research use only
Adapalene Retinoid Receptor agonist
Cat.No.S1276
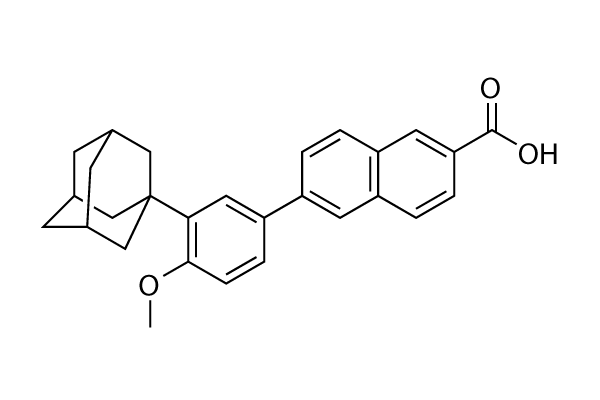
Chemical Structure
Molecular Weight: 412.52
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other Retinoid Receptor Inhibitors | TTNPB (Arotinoid acid) Tamibarotene AM580 SR 11237 UVI 3003 Fenretinide BMS493 AR7 Palovarotene CD1530 |
Solubility
|
In vitro |
DMSO
: 17 mg/mL
(41.21 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 412.52 | Formula | C28H28O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 106685-40-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CD-271 | Smiles | COC1=C(C=C(C=C1)C2=CC3=C(C=C2)C=C(C=C3)C(=O)O)C45CC6CC(C4)CC(C6)C5 | ||
Mechanism of Action
| Targets/IC50/Ki |
RARγ
RARβ
RXR
|
|---|---|
| In vitro |
Adapalene binds to retinoic acid receptors found predominantly in the terminal differentiation zone of epidermis and is more active than tretinoin in modulating cellular differentiation. This compound shows greatest affinity for the subtype PARγ, found predominantly in the epidermis. It is more active than indomethacin, betamethasone valerate, tretinoin, isotretinoin or etretinate in inhibiting lipoxygease activity, but it has little activity against cyclo-oxygenase. This chemical time- and dose-dependently suppresses DNA synthesis and induces apoptosis in Colon carcinoma cell lines CC-531, HT-29 and LOVO as well as human foreskin fibroblasts. It shows significantly more effective antiproliferative and proapoptotic effects than 9-cis-retinoic acid (CRA), showing remarkable effects even at 10 μM. This compound disrupts DeltaPsi(m) and induces caspase-3 activity in responsive tumor cells.
|
| In vivo |
Adapalene produces a dose-related reduction in the number of epidermal comedones and an increase in comedo profile and epidermal thickness in the rhino mice. This compound results in a decreased expression of TLR-2 and IL-10 in explants of normal skin and explants of acne. It can modulate the epidermal immune system by increasing the CD1d expression and by decreasing the IL-10 expression by keratinocytes, and these modulations can increase the interactions between dendritic cells and T lymphocytes and could strengthen the antimicrobial activity against Propionibacterium acnes.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02620813 | Completed | Acne Vulgaris |
University of California Davis |
October 2015 | Early Phase 1 |
| NCT02442817 | Completed | Schizophrenia |
University of Nevada Reno|Augusta University |
March 2 2015 | Phase 4 |
| NCT01485367 | Unknown status | Senile Purpura |
Multispecialty Aesthetic Clinical Research Organization|Galderma R&D |
December 2011 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































