research use only
GSK2879552 Dihydrochloride LSD1 Inhibitor
Cat.No.S7796
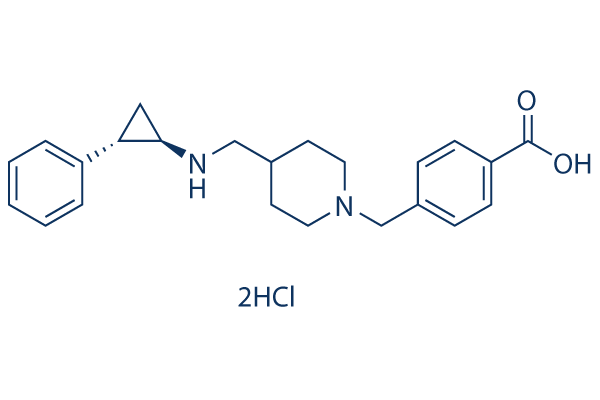
Chemical Structure
Molecular Weight: 437.4
Quality Control
| Related Targets | HDAC JAK BET Histone Methyltransferase PKC PARP HIF PRMT EZH2 AMPK |
|---|---|
| Other Histamine Receptor Inhibitors | JNJ-7777120 Ebastine Mianserin HCl Astemizole Ciproxifan Maleate Lafutidine Mizolastine Dimethindene maleate Rupatadine Levodropropizine |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| THP-1 | Function assay | 0, 7, 47, and 3000 nM | 1 day | a dose-dependent increase in CD11b and CD86 expression with average EC50 values of 23 +/- 4 nM | 30514804 | |
| MOLM-13 | Proliferation assay | 6 days | dose responsive decrease in BrdU signal, EC50 = 1.9 +/- 0.9 nM | 30514804 | ||
| MV4-11 | Antiproliferative assay | 240 hrs | Antiproliferative activity against human MV4-11 cells after 240 hrs by MTS assay, IC50=1.16μM | 30713023 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
Water : 44 mg/mL
DMSO
: 29 mg/mL
(66.3 mM)
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 437.4 | Formula | C23H28N2O2.2HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1902123-72-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CN(CCC1CNC2CC2C3=CC=CC=C3)CC4=CC=C(C=C4)C(=O)O.Cl.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
LSD1
(Cell-free assay) 1.7 μM(Ki)
|
|---|---|
| In vitro |
In 165 cell lines, GSK2879552 inhibits the growth of 9/28 small cell lung carcinoma (SCLC) lines and 20/29 AML lines ranged from 40% to 100%. The subset of SCLC lines and primary samples that undergo growth inhibition in response to GSK2879552 exhibit DNA hypomethylation of a signature set of probes.
|
| Kinase Assay |
LSD1 enzyme assay
|
|
LSD1 activity was measured using a horseradish peroxidase (HRP) coupled assay with amplex red as an electron donor. The formation of product over time is measured using fluorescence intensity, Ex 531 nm and Em 595 nm, in a PerkinElmer EnVision plate reader. Final assay conditions are: 5 nM LSD1, 2.5 μM H3K4me2 peptide, 50 mM HEPES pH 7, 1 U/ml of HRP, 1 mM CHAPS, 0.03% dBSA and 10 μM amplex red.
|
|
| In vivo |
In SCLC xenograft bearing mice, GSK2879552 (1.5 mg/kg, p.o.) demonstrates tumor growth inhibition by 17%-83%.
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
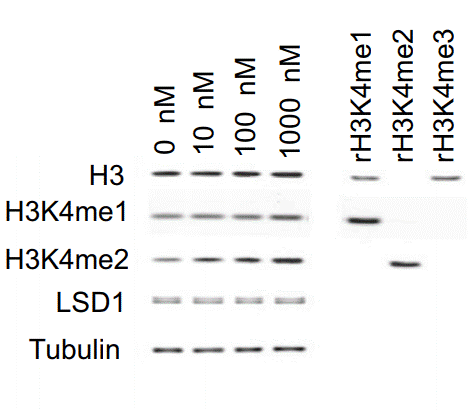
| Western blot | H3 / H3K4me1 / H3K4me2 / LSD1 |
|
26175415 |
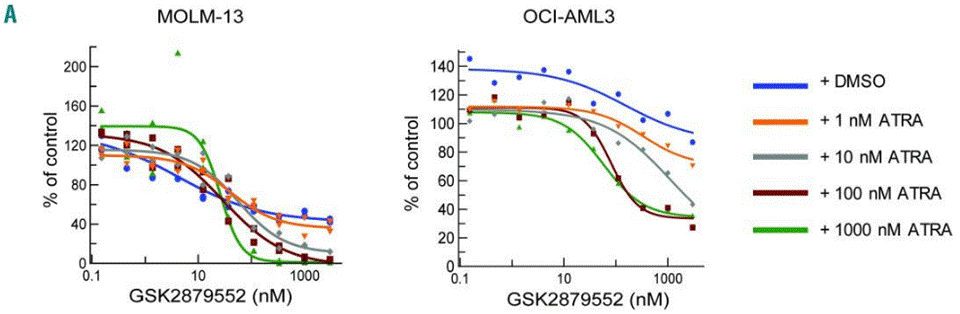
| Growth inhibition assay | Cell viability |
|
30514804 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02929498 | Terminated | Myelodysplastic Syndrome|Myelodysplastic Syndromes |
GlaxoSmithKline|Parexel |
July 31 2017 | Phase 1|Phase 2 |
| NCT02177812 | Terminated | Leukaemia Myelocytic Acute |
GlaxoSmithKline |
August 27 2014 | Phase 1 |
| NCT02034123 | Terminated | Carcinoma Small Cell |
GlaxoSmithKline |
February 4 2014 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































