- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Ebastine Histamine Receptor antagonist
Cat.No.S4262
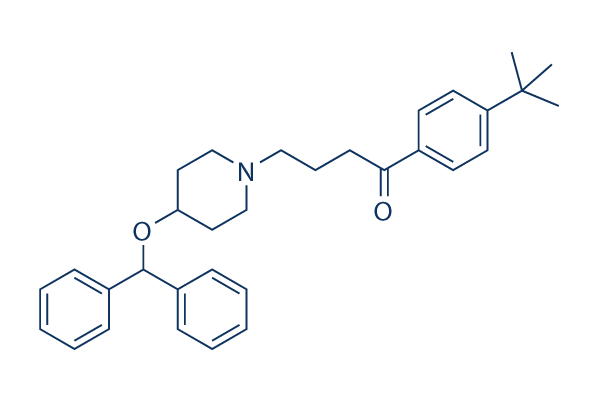
Chemical Structure
Molecular Weight: 469.66
Jump to
Quality Control
Batch:
Purity:
99.86%
99.86
Solubility
|
In vitro |
DMSO
: 93 mg/mL
(198.01 mM)
Ethanol : 93 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 469.66 | Formula | C32H39NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 90729-43-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LAS-W 090,RP64305 | Smiles | CC(C)(C)C1=CC=C(C=C1)C(=O)CCCN2CCC(CC2)OC(C3=CC=CC=C3)C4=CC=CC=C4 | ||
Mechanism of Action
| Targets/IC50/Ki |
Histamine H1 receptor
|
|---|---|
| In vitro |
Ebastine at concentrations approximating those found in plasma under certain conditions suppresses in a voltage-independent manner the I(Kr) (Kd = 0.14 μM, maximum block 74%) more effectively than the slowly delayed rectifier K+ current (I(Ks)) (Kd = 0.8 μM, maximum block 60%) in guinea pig ventricular myocytes. This compound also suppresses IKr in HERG-expressing X. laevis oocytes with the K d value of 0.3 μM and a maximal block of 46% at 3 μM. It produces negligible effect on rat transient K+ current at concentrations of <100 nM. This chemical is shown to block the release of anti-IgE-induced prostaglandin D2 (PGD2) and leukotriene C4/D4 from human nasal polyp cells (IC30 values of 2.57 and 9.6 μM, respectively) and to inhibit the release of cytokines. It is a novel histamine H1 receptor antagonist that combines potency with a rapid onset (fast absorption) and long duration (slow elimination) of action, at least partially mediated via the formation of an acid metabolite (carebastine) that is even more potent as an antihistamine. This agent has negligible activity against acetylcholine (no atropine-like adverse effects on secretions and visual accommodation) and only poorly penetrates the blood-brain barrier (no sedative adverse effects). It is without effects on the central nervous and cardiovascular systems, even after oral administration of high doses, and does not interact pharmacologically with a wide range of other drugs covering most areas of potential coadministration. This compound shows clear selectivity for histamine H1 as opposed to H2 receptors, has moderate activity against other potential mediators of allergic phenomena such as leukotriene C4 and platelet-activating factor, and is clearly effective against anaphylactic reactions resulting from exposure of suitably sensitised tissues or animals to antigen. |
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































