research use only
GSK2256098 FAK inhibitor
Cat.No.S8523
Chemical Structure
Molecular Weight: 414.89
Quality Control
| Related Targets | EGFR VEGFR JAK PDGFR FGFR Src HIF FLT FLT3 HER2 |
|---|---|
| Other FAK Inhibitors | Defactinib (VS-6063) PF-562271 (VS-6062) PF-573228 VS-4718 (PND-1186) PF-562271 HCl PF-562271 Besylate TAE226 (NVP-TAE226) PF-431396 Y15 Ifebemtinib (IN10018, BI-853520) |
Solubility
|
In vitro |
DMSO
: 83 mg/mL
(200.05 mM)
Ethanol : 83 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 414.89 | Formula | C20H23ClN6O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1224887-10-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GTPL7939 | Smiles | CC1=NN(C(=C1)NC2=NC=C(C(=C2)NC3=CC=CC=C3C(=O)NOC)Cl)C(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
FAK
0.4 nM(Ki)
|
|---|---|
| In vitro |
GSK2256098 has been developed to inhibit FAK activity through targeting the phosphorylation site of FAK, tyrosine (Y) 397. After a 30-min incubation, this compound inhibits FAK activity or Y397 phosphorylation in cancer cell lines, OVCAR8 (ovary), U87MG (brain), and A549 (lung), at IC50 values of 15, 8.5 and 12 nM, respectively. In addition, the data suggests that cellular inhibition of FAK by this chemical can occur as early as 30 min in cultured cells and lasts up to 12 hours in mouse tumor xenografts. Its inhibition of FAK kinase activity can decrease Akt and ERK activity. PI3K/Akt and ERK signaling contributes to cell survival, implying a pharmacological value of this compound in attenuation of abnormal survival pathways in specific types of PDAC cells. It can promote apoptosis in L3.6P1 cells through caspase-9/PARP-related pathways. It attenuates abnormal growth and aberrant motility of PDAC cells in a FAK specific manner. This compound also inhibits growth, migration, and invasion and induces apoptosis in a subset of GBM cell lines. |
| In vivo |
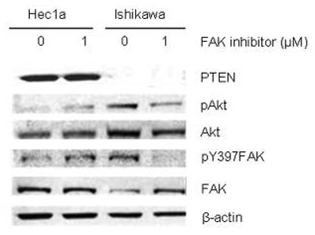
Pharmacokinetic (PK) studies in mice and rats with an intact blood brain barrier indicate that the penetration of GSK2256098 into the CNS is poor. However, it achieves concentrations in tumor of patients with GBM(glioblastoma) exceeding those associated with preclinical activity. This compound has an acceptable safety profile, has evidence of target engagement at doses at or below the MTD (maximum tolerated dose), and has clinical activity in patients with mesothelioma, particularly those with merlin loss. In the Ishikawa orthoptopic murine model, treatment with this compound results in lower tumor weights and fewer metastases than mice inoculated with Hec1A cells. Tumors treated with this chemical have lower microvessel density (CD31), less cellular proliferation (Ki67), and higher apoptosis (TUNEL) rates in the Ishikawa model when compared to the Hec1a model. It may be therapeutically beneficial to patients with PTEN-mutant uterine cancer, and PTEN represents a potential predictive biomarker. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-CHK1/ CHK1 / γH2AX / c-PARP PTEN / p-AKT / AKT / pY397-FAK / FAK |
|
26295308 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02551653 | Completed | Hypertension Pulmonary |
GlaxoSmithKline |
November 17 2015 | Phase 1 |
| NCT01938443 | Completed | Cancer|Neoplasms |
GlaxoSmithKline |
November 18 2013 | Phase 1 |
| NCT01138033 | Completed | Cancer |
GlaxoSmithKline |
July 27 2010 | Phase 1 |
| NCT00996671 | Completed | Cancer |
GlaxoSmithKline |
November 6 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































