research use only
ASP2215 (Gilteritinib) FLT3/AXL inhibitor
Cat.No.S7754
Chemical Structure
Molecular Weight: 552.71
Quality Control
Products Often Used Together with ASP2215 (Gilteritinib)
| Related Targets | EGFR VEGFR PDGFR FGFR c-Met Src MEK CSF-1R HER2 c-Kit |
|---|---|
| Other FLT3 Inhibitors | UNC2025 Crenolanib (CP-868596) Dovitinib (TKI-258) Dovitinib (TKI258) Lactate monohydrate Tandutinib (MLN518) KW-2449 ENMD-2076 AST-487 (NVP-AST487) TCS 359 FLT3-IN-2 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MV4-11 cells | Cell cycle assay | 1, 3, 10, and 30 nM | 24 h | The mean proportion of MV4-11 cells in G1 phase were significantly increased at gilteritinib concentrations of 3 (69.0%; P<0.01) and 10 nM (70.7%; P<0.001). | 31069015 | |
| MOLM-13 cells | Apoptosis assay | 1, 3, 10, 30, and 100 nM | 48 h | significant increases in the percentage of annexin V-positive cells at concentrations of 30 nM (32.0%) and 100 nM (52.4%) versus control (4.1%) | 31069015 | |
| 32D/TKD cells | Function assay | 50 nM | 6 h | Inhibiton of the phosphorylation of Akt on T308 | 29507660 | |
| TF-1 cells | Function assay | 0, 20, 80, 200 and 500 nM | 1 h | gilteritinib has an IC50 against wild-type c-Kit of 102 nM | 27908881 | |
| BA/F3 | Function assay | Inhibition Assay: A recombinant retrovirus was created from expression plasmid FLAG-EML4-ALKv1/pMX-iresCD8 in which cDNA for EMLA-ALK fusion protein v1 was integrated, and injected into mouse lymphoid cell line BA/F3 cells. Using a magnetic bead reagent f, IC50 = 0.0015 μM. | ChEMBL | |||
| BA/F3 | Function assay | Inhibition Assay: A recombinant retrovirus was created from expression plasmid FLAG-EML4-ALKv1/pMX-iresCD8 in which cDNA for EMLA-ALK fusion protein v1 was integrated, and injected into mouse lymphoid cell line BA/F3 cells. Using a magnetic bead reagent f, IC50 = 0.0015 μM. | ChEMBL | |||
| Vero | Antiviral assay | 24 hr | Antiviral activity against SARS-CoV-2 (viral titer) measured by plaque assay in Vero cells at MOI 0.0125 after 24 hr, IC50 = 6.76 μM. | ChEMBL | ||
| Vero | Cell viability assay | 72 hr | Cell viability measured by CellTiter-Glo assay in Vero cells at MOI 0.05 after 72hr, CC50 = 37.16 μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 4 mg/mL
(7.23 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 552.71 | Formula | C29H44N8O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1254053-43-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCC1=C(N=C(C(=N1)C(=O)N)NC2=CC(=C(C=C2)N3CCC(CC3)N4CCN(CC4)C)OC)NC5CCOCC5 | ||
Mechanism of Action
| Targets/IC50/Ki |
FLT3
(Cell-free assay) 0.29 nM
Axl
(Cell-free assay) 0.73 nM
|
|---|---|
| In vitro |
Gilteritinib (ASP2215) demonstrates potent inhibitory activity against the internal tandem duplication (FLT3-ITD) and FLT3-D835Y point mutations in cellular assays using MV4-11 and MOLM-13 cells as well as Ba/F3 cells expressing mutated FLT3. It decreases the phosphorylation levels of FLT3 and its downstream targets in both cellular and animal models. This compound inhibits the activity of eight of the 78 tested kinases by over 50% at concentrations of either 1 nM (FLT3, LTK, ALK, and AXL) or 5 nM (TRKA, ROS, RET, and MER). Treatment with it for 48h results in an induction of apoptosis in MV4-11 cells as determined by an increase in annexin V-positive cells. It also decreases the expression of anti-apoptotic proteins such as MCL-1, BCL2L10, and survivin, which are reported to be important in chemotherapy sensitivity, following 24h treatment.
|
| In vivo |
In vivo, gilteritinib (ASP2215) is distributed at high levels in xenografted tumors after oral administration. The decreased FLT3 activity and high intratumor distribution of this compound translates to tumor regression and improved survival in xenograft and intra-bone marrow transplantation models of FLT3-driven AML. This antitumor activity is associated with a durable inhibition of phospho-FLT3 and phospho-STAT5. Furthermore, treatment with it decreases the leukemic burden and prolongs survival in a mouse IBMT model. No overt toxicity is seen in mouse models treated with gilteritinib.
|
References |
|
Applications
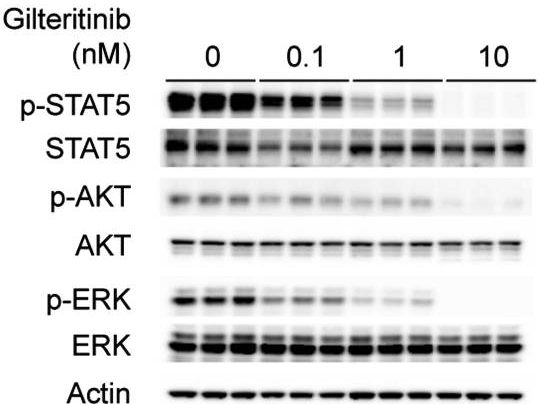
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-STAT5 / STAT5 / p-AKT / AKT / p-ERK / ERK p-c-kit / c-kit p-FLT3(Y591) / FLT3 |
|
28516360 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06022003 | Recruiting | AML Adult|Refractory AML|Relapsed Adult AML|FLT3-TKD Mutation|FLT3-ITD |
French Innovative Leukemia Organisation|Acute Leukemia French Association |
January 13 2024 | Phase 2 |
| NCT05520567 | Recruiting | Acute Myeloid Leukemia (AML)|FLT3-mutated Acute Myeloid Leukemia |
Astellas Pharma Global Development Inc.|Astellas Pharma Inc |
January 27 2023 | Phase 1|Phase 2 |
| NCT05791890 | Active not recruiting | Acute Myeloid Leukemia |
University of Rome Tor Vergata |
May 31 2022 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































