research use only
Cyclophosphamide Monohydrate Alkylating Agent
Cat.No.S2057
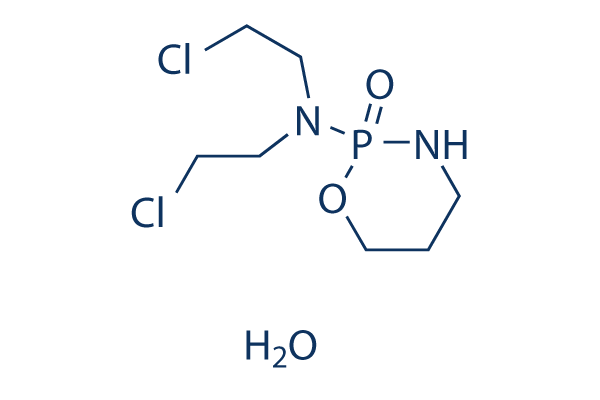
Chemical Structure
Molecular Weight: 279.1
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase |
|---|---|
| Featured Inhibitors | Y-27632 Dihydrochloride SB431542 CHIR-99021 (Laduviglusib) RMC-7977 RMC-6236 (Daraxonrasib) MRTX1133 MG132 Z-VAD-FMK VT3989 IAG933 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| HL60 cells | Cytotoxicity assay | Cytotoxicity against human HL60 cells by MTT assay, IC50=8.79 μM | 20850303 | |||
| BALB/c 3T3 cells | Cytotoxicity assay | In vitro cytotoxicity was evaluated in mouse embryo BALB/c 3T3 cells, IC50=37.6 μM | 9873412 | |||
| BALB/c 3T3 | Cytotoxicity assay | In vitro cytotoxicity against BALB/c 3T3 cells, IC50 = 37.6 μM. | 7877150 | |||
| T-cells | Immunosuppressive assay | 100 mg/kg | 8 days | Immunosuppressive activity against MAV-1 infected BALB/c mouse assessed as T cells suppression at 100 mg/kg, ip treated 8 days before infection for 4 weeks measured 14 days post infection by flow cytometry | 18268085 | |
| B-cells | Immunosuppressive assay | 100 mg/kg | 8 days | Immunosuppressive activity against MAV-1 infected BALB/c mouse assessed as B cells suppression at 100 mg/kg, ip treated 8 days before infection for 4 weeks measured 14 days post infection by flow cytometry | 18268085 | |
| U87 MG | Antitumor assay | 80 mg/kg | Antitumor activity in human U87 MG cells xenografted mouse at 80 mg/kg, iv administered in Q2D x 5 schedule | 18450456 | ||
| MX1 | Antitumor assay | 120 mg/kg | up to 3 weeks | Antitumor activity against human MX1 cells xenografted in nude mouse adjuvant model assessed as increase in mouse survival time at 120 mg/kg, po BID measured up to 3 weeks | 20726512 | |
| U87MG | Anticancer assay | 80 mg/kg | 6 days | Anticancer activity against human U87MG cells xenografted in athymic mouse assessed as tumor suppression at 80 mg/kg, iv Q2D for 6 days | 21106377 | |
| NCI-H522 | Antitumor assay | 50 mg/kg | 28 days | Antitumor activity against human NCI-H522 cells xenografted in Balb/c nude mouse assessed as tumor growth inhibition at 50 mg/kg, ig administered once daily for 28 days relative to control | 28092860 | |
| U2932 | Antitumor assay | 50 mg/kg | 5 consecutive days | Antitumor activity against human U2932 cells xenografted in SCID mouse assessed as reduction in tumor burden at 50 mg/kg, ip administered for 5 consecutive days measured up to day 40 post cell injection | 28605593 | |
| MDA-MB-231 | Cytotoxicity assay | 48 hr | Cytotoxicity against Homo sapiens (human) MDA-MB-231 cells after 48 hr by MTT assay, IC50 = 0.09 μM. | ChEMBL | ||
| K562 | Cytotoxicity assay | 48 hr | Cytotoxicity against Homo sapiens (human) K562 cells after 48 hr by MTT assay, IC50 = 0.15 μM. | ChEMBL | ||
| K562 | Antiproliferative assay | 48 hr | Antiproliferative activity against Homo sapiens (human) K562 cells after 48 hr by MTT assay, IC50 = 0.153 μM. | ChEMBL | ||
| MCF7 | Cytotoxicity assay | 48 hr | Cytotoxicity against Homo sapiens (human) MCF7 cells after 48 hr by SRB assay, IC50 = 10 mM. | 19372630 | ||
| HepG2 | Cytotoxicity assay | 48 hr | Cytotoxicity against Homo sapiens (human) HepG2 cells after 48 hr by MTT assay, IC50 = 0.24 μM. | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 55 mg/mL
(197.06 mM)
Water : 55 mg/mL Ethanol : 55 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 279.1 | Formula | C7H15Cl2N2O2P.H2O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 6055-19-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NSC-26271 Monohydrate | Smiles | C1CNP(=O)(OC1)N(CCCl)CCCl.O | ||
Mechanism of Action
| In vitro |
Cyclophosphamide (CY) is a chemotherapeutic agent with a dose-dependent, bimodal effect on the immune system. Cyclophosphamide treatment enhances apoptosis and decreases homeostatic proliferation of regulatory T cells. Cyclophosphamide downregulates the expression of GITR and FoxP3, which are involved in the suppressive activity of T(REGs). Cyclophosphamide increases CYP3A4, CYP2C8, and CYP2C9 protein levels in primary human hepatocyte cultures, which thereby enhances their own rates of 4-hydroxylation in the cultured hepatocytes. Cyclophosphamide has produced mutations in base-pair substituting strains of Salmonella tryphimurium in the presence of metabolic activation, but it has been shown to be negative in the E. coli chromotest. Cyclophosphamide has been shown to produce gene mutations, chromosome aberrations, micronuclei and sister chromatid exchanges in a variety of cultured cells in the presence of metabolic activation as well as sister chromatid exchanges without metabolic activation. |
|---|---|
| In vivo |
Cyclophosphamide has also produced chromosome damage and micronuclei in rats, mice and Chinese hamsters, and gene mutations in the mouse spot test and in the transgenic lacZ construct of Muta Mouse. Cyclophosphamide, when given in a defined sequence with a GM-CSF-secreting, neu-expressing whole-cell vaccine, enhances the vaccine's potential to delay tumor growth in neu transgenic mice. Cyclophosphamide mediates its effects by enhancing the efficacy of the vaccine rather than via a direct cytolytic effect on cancer cells. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | PTEN / pAkt / Caspase 3 / GAPDH Caspase 3 / Cleaved-Caspase 3 / Cleaved-PARP / Tubulin Nuclear AIF / Cytosolic AIF / TBP / GAPDH LC3B I / LC3B II / GAPDH |
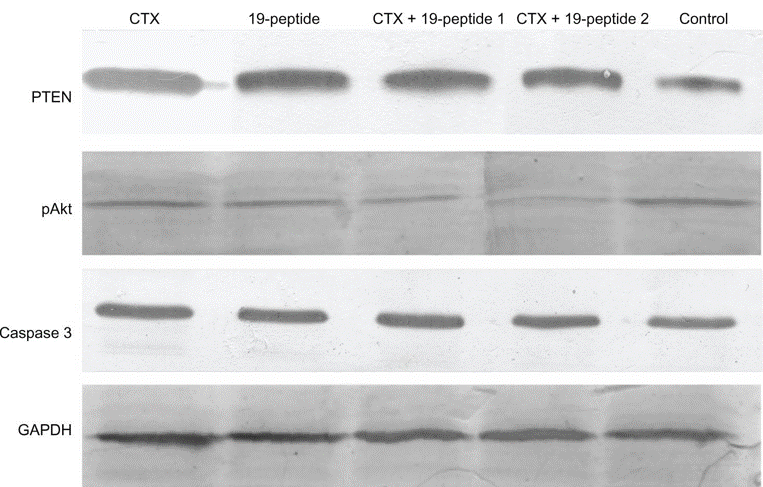
|
23874108 |
| Growth inhibition assay | Cell Viability Tumor Volume Tumor Volume |
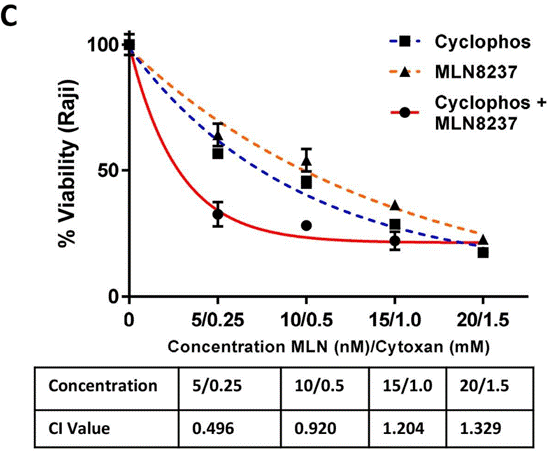
|
31429028 |
| IHC | tumor cell invasion TUNEL / Caspase 3 PTEN / pAkt / PCNA ovary of mice Caspase-3 |
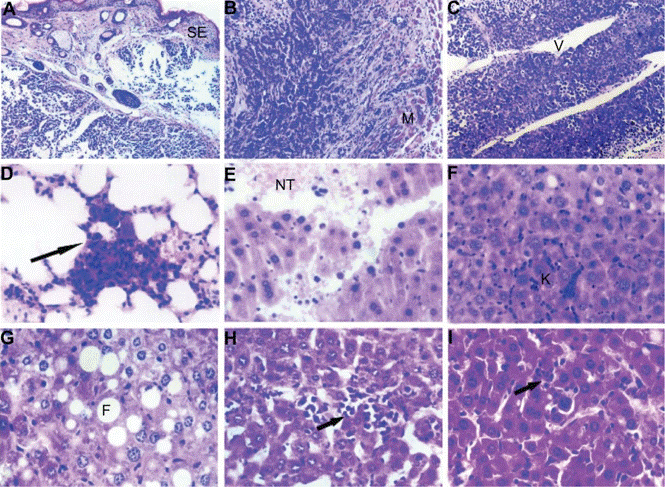
|
23874108 |
| Immunofluorescence | pAKT / pERK Ki-67 / UPK3 / KRT5 / pERK Ki-67 / KRT14 / KRT5 autophagy levels |
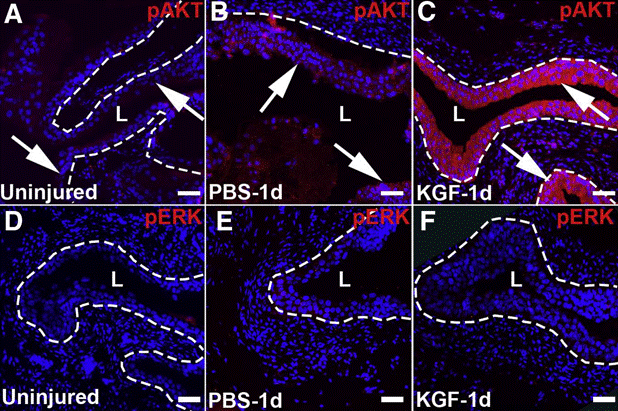
|
31654636 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06186700 | Active not recruiting | Breast Cancer Female |
Mansoura University |
December 25 2023 | Phase 2 |
| NCT06085742 | Recruiting | Breast Cancer |
University of Illinois at Chicago |
November 22 2023 | Phase 2 |
| NCT05800041 | Not yet recruiting | Gout Tophus |
RenJi Hospital|Westlake Therapeutics |
April 10 2023 | Early Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
Why does it show no activity in vitro assays?
Answer:
The activity of this compound in vitro is controversial and has not been fully determined. It is a prodrug and may need Cytochrome P450 to convert it to the active form: 4-hydroxy cyclophosphamide. It is widely used in vivo, if you are going to use it in vitro, you may need to supplement Cytochrome P450 exogenously.






































