research use only
Betahistine 2HCl Histamine Receptor inhibitor
Cat.No.S3176
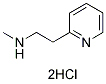
Chemical Structure
Molecular Weight: 209.12
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Histamine Receptor Inhibitors | GSK2879552 Dihydrochloride JNJ-7777120 Ebastine Mianserin HCl Astemizole Ciproxifan Maleate Lafutidine Mizolastine Dimethindene maleate Rupatadine |
Solubility
|
In vitro |
DMSO
: 38 mg/mL
(181.71 mM)
Water : 38 mg/mL Ethanol : 38 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 209.12 | Formula | C8H12N2.2HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 5579-84-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | PT-9 | Smiles | CNCCC1=CC=CC=N1.Cl.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Histamine H3 receptor
1.9 μM
|
|---|---|
| In vitro |
Betahistine progressively enhances cAMP formation with a maximal effect, observed up to 10 nM, in CHO(H3R) cells incubated with 3 μM forskolin. In contrast, at concentrations higher than 10 nM betahistine progressively inhibits cAMP formation in CHO(H3R) cells incubated with 3 μM forskolin. Betahistine progressively reduces A23187-evoked [3H]arachidonic acid release (EC50=0.1 nM) with a maximal effect, observed up to 30 nM A23187-evoked [3H]arachidonic acid release from CHO(H3R) cells. Betahistine progressively enhanced the release of A23187-evoked [3H]arachidonic acid from CHO(H3R) cells at concentrations higher than 30 nM.
|
| In vivo |
Betahistine (< 30 mg/kg) increases t-MeHA levels in a dose-dependent manner with an ED50 of 2 mg/kg and a maximal effect of ∼35% reached at 30 mg/kg in mouse brain. Betahistine (16 mg twice per day for 3 months) has a significant effect on the frequency, intensity and duration of vertigo attacks, associated symptoms and the quality of life also are significantly improved in patients with Meniere's disease. Betahistine-dihydrochloride (16 mg tid and 48 mg tid) shows that the number of attacks per month decreased in both doses over time in Meni鑢e's disease. Betahistine (50 mg/kg) treatment induces symmetrical changes with up-regulation of histidine decarboxylase mRNA in the tuberomammillary nucleus and reduction of [3H]N-alpha-methylhistamine labeling in both the tuberomammillary nucleus, the vestibular nuclei complex and nuclei of the inferior olive in brain sections of cats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT00852956 | Completed | Healthy |
OBEcure Ltd. |
February 2009 | Phase 1 |
| NCT00585585 | Terminated | Recurrent Major Depressive Disorder With Atypical Features |
University of Cincinnati |
July 2007 | Phase 2 |
| NCT00459992 | Completed | Obesity|Overweight|Overnutrition |
Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)|National Institutes of Health Clinical Center (CC) |
April 10 2007 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































