research use only
Benzocaine Sodium Channel inhibitor
Cat.No.S4210
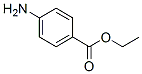
Chemical Structure
Molecular Weight: 165.19
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Sodium Channel Inhibitors | Camostat Mesilate A-803467 cariporide Veratramine Bulleyaconi cine A Vinpocetine Tenapanor PF-06869206 Sparteine (-)-Sparteine Sulfate |
Solubility
|
In vitro |
DMSO
: 33 mg/mL
(199.76 mM)
Ethanol : 33 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 165.19 | Formula | C9H11NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 94-09-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | ethyl 4-aminobenzoate | Smiles | CCOC(=O)C1=CC=C(C=C1)N | ||
Mechanism of Action
| Targets/IC50/Ki |
Sodium channel
|
|---|---|
| In vitro |
Benzocaine blocks µ1 wild-type Na+ currents in a dose-dependent manner with IC50 of 0.8 mM in HEK293T cells. This compound (1 mM) blocks about 55% of wild-type Na+ current but about 95% of µ1-N1584A mutant current. It (1 mM) blocks about 55% of wild-type µ1 currents, but about 80% of µ1-I1575A mutant current. This chemical results in a biphasic (protective/inductive) concentration-dependent hemolytic effect upon rat erythrocytes, with an effective Benzocaine:lipid molar ratio in the membrane for protection (RePROT), onset of hemolysis (ReSAT) and 100% membrane solubilization (ReSOL) of 1.0:1, 1.1:1 and 1.3:1, respectively. It and 4-hydroxybenzoate interact with the open and inactivated channels during repetitive pulses, but during the interpulse the complex dissociates too fast to accumulate sufficient use-dependent block of Na+ currents. This compound (500 μM) reduces the peak and steady-state currents and increases the amplitude of the inactivating component from 21.7% to 30.2% (n=7, P<0.05), so that benzocaine-induced block at the end of pulses to +60 mV averaged 30.9% (n=7). It (500 μM) significantly accelerates the initial phase of deactivation (τf=27.2±2.6 ms, n=7, P<0.01), but does not modify the slow phase of tail current decline. This chemical binds with high affinity to an intracellular binding site to produce 'agonist' effects and to a low affinity subsite, which is also located in the inner mouth, to produce the blocking effects. It and extracellular K(+) interact to modify the voltage-dependence of channel opening. |
| In vivo |
Benzocaine is absorbed rapidly and similarly through both viable and nonviable skin of the hairless guinea pig, the absorption of the two acidic compounds, benzoic acid and PABA, is greater through nonviable skin. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06165432 | Completed | Pain |
B.P. Koirala Institute of Health Sciences |
March 19 2021 | Phase 4 |
| NCT03116737 | Completed | Pain|Acute Otitis Media |
Lachlan Pharma Holdings |
January 3 2017 | Phase 3 |
| NCT02092454 | Completed | Pain|Acute Otitis Media |
Otic Therapy LLC |
September 2013 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































