- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Asciminib (ABL001) STAMP Inhibitor
Cat.No.S8555
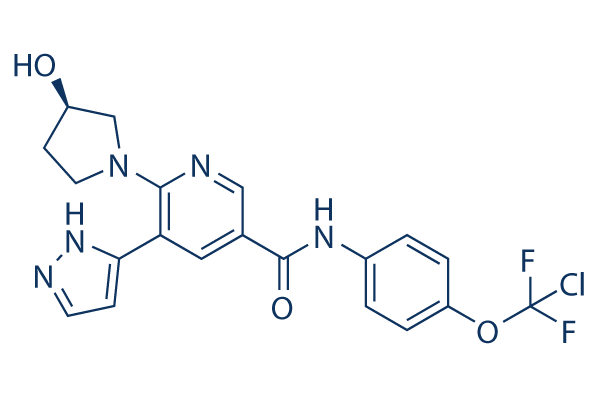
Chemical Structure
Molecular Weight: 449.84
Jump to
Quality Control
Batch:
Purity:
99.88%
99.88
Solubility
|
In vitro |
DMSO
: 89 mg/mL
(197.84 mM)
Ethanol : 89 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 449.84 | Formula | C20H18ClF2N5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1492952-76-7 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1CN(CC1O)C2=C(C=C(C=N2)C(=O)NC3=CC=C(C=C3)OC(F)(F)Cl)C4=CC=NN4 | ||
Mechanism of Action
| Targets/IC50/Ki |
Abl1
(Cell-free assay) 0.45 nM
|
|---|---|
| In vitro |
Asciminib (ABL001) is a potent, selective BCR-ABL inhibitor that maintains activity across most mutations, including T315I, with a distinct, allosteric mechanism of action. It binds at a regulatory site typically occupied by a myristoyl group in wild-type ABL and inhibits ABL kinase activity through a mechanism distinct from catalytic site inhibitors. This compound binds to a pocket on the BCR-ABL kinase domain that is normally occupied by the myristoylated N-terminus of ABL1. Upon fusion with BCR, this myristoylated N-terminus that serves to autoregulate ABL1 activity is lost. ABL001 functionally mimics the role of the myristoylated N-terminus by occupying its vacant binding site and restores the negative regulation of the kinase activity. It selectively inhibits the growth of chronic myelogenous leukemia (CML) and Ph+ ALL cells with potencies ranging from 1-10 nM range while BCR-ABL-negative cell lines remained unaffected at concentrations 1000-fold higher. NMR and biophysical studies confirm that it binds potently (dissociation constant (Kd) = 0.5-0.8 nM) and selectively to the myristoyl pocket of ABL1 and induces the inactive C-terminal helix conformation. This compound lacks activity against more than 60 kinases, including SRC and is similarly inactive against G-protein-coupled receptors, ion channels, nuclear receptors and transporters. Thus, it has high selectivity. |
| In vivo |
In the KCL-22 mouse xenograft model, Asciminib (ABL001) displays potent anti-tumor activity with complete tumor regression observed and a clear dose-dependent correlation with pSTAT5 inhibition. It has moderate oral absorption, volume of distribution and half-life across all species. This compound as a single agent induces clinical anti-tumour activity and is well tolerated to date in a heavily pre-treated subgroup of patients with chronic myelogenous leukemia. As for the pharmacokinetics, pharmacodynamics and efficacy, the CL (clearance) are 12, 16 and 6 mL/min/kg in mice, rats and dogs after a single iv dose of 1mg/kg, 2mg/kg and 1mg/kg, respectively. In mouse and dog, the T1/2term are 1.1 and 3.7 h after a single i.v. dose at 1 mg/kg. In Rat, the T1/2term is 2.7 h after a single i.v. dose at 2 mg/kg. The oral bioavailability in mouse and rat are 35% and 27% respectively when dosed at 30 mg/kg p.o. While in dogs the oral BA is 111% (15 mg/kg, p.o). |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06409936 | Not yet recruiting | CML Chronic Phase|Chronic Myeloid Leukemia Chronic Phase|Chronic Myeloid Leukemia BCR/ABL-Positive|Chronic Myeloid Leukemia |
Gruppo Italiano Malattie EMatologiche dell''Adulto|Fundacion Espanola para la Curacion de la Leucemia Mieloide Cronica |
September 2024 | Phase 2 |
| NCT06092879 | Recruiting | Chronic Myeloid Leukemia |
Novartis Pharmaceuticals|Novartis |
March 6 2024 | -- |
| NCT06211153 | Completed | Chronic Myeloid Leukemia With T315I Mutation |
Novartis Pharmaceuticals|Novartis |
December 31 2021 | -- |
| NCT04795427 | Active not recruiting | Leukemia Chronic Myelogenous |
Novartis Pharmaceuticals|Novartis |
December 6 2021 | Phase 2 |
| NCT04492033 | Active not recruiting | P1b: Advanced Solid Tumors|P2: Biliary Tract Cancer |
Handok Inc.|Compass Therapeutics|ABL Bio Inc. |
June 22 2020 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































