research use only
Ranitidine Hydrochloride Histamine Receptor antagonist
Cat.No.S1801
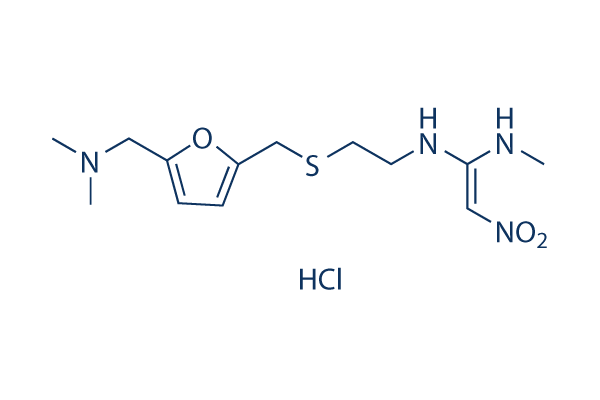
Chemical Structure
Molecular Weight: 350.86
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Histamine Receptor Inhibitors | GSK2879552 Dihydrochloride JNJ-7777120 Ebastine Mianserin HCl Astemizole Ciproxifan Maleate Lafutidine Mizolastine Dimethindene maleate Rupatadine |
Solubility
|
In vitro |
DMSO
: 70 mg/mL
(199.5 mM)
Water : 70 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 350.86 | Formula | C13H22N4O3S.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 66357-59-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | AH19065 | Smiles | CNC(=C[N+](=O)[O-])NCCSCC1=CC=C(O1)CN(C)C.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Histamine H2 receptor
|
|---|---|
| In vitro |
Ranitidine sensitizes hepatocytes to killing by cytotoxic products from activated neutrophils, whereas Famotidine lacks this ability. Ranitidine inhibits the production of tumor necrosis factor-alpha (TNF-alpha) in monocytes stimulated with lipopolysaccharide in vitro. Ranitidine reduces the Kel of morphine dose-dependently with a maximum effect of 50%, and increases the relative concentration of morphine-6-glucuronide to morphine-3-glucuronide in isolated guinea pig hepatocytes. Ranitidine gradually decreases the morphine-3-glucuronide/morphine-6-glucuronide ratio by up to 21%.
|
| In vivo |
Ranitidine results in liver injury as evidence by increased in serum alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase activities within 6 hours after Ranitidine administration in rats. Ranitidine inhibits hepatic ischemia/reperfusion-induced increase in hepatic tissue levels of TNF-alpha, cytokine-induced neutrophil chemoattractant, and hepatic accumulation of neutrophils in rats. Ranitidine cotreatment enhances LPS-induced coagulation prior to liver injury, and anticoagulants reduce liver damage in LPS/RAN-treated rats. Ranitidine /LPS-treated rats results in the formation of fibrin clots in liver sinusoids, and prevention of fibrin deposition associated with reduced hepatocellular injury. Ranitidine cotreatment enhances the LPS-induced TNF increase before the onset of hepatocellular injury in rats. Ranitidine displays anxiolytic effects in the elevated plus-maze as indicated by an increase in time spent in the open arms, more open-arm scanning and more end-excursions in rats.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05399927 | Completed | Music Therapy|Cardiovascular Diseases|Critical Care|Emergencies |
Universidad Miguel Hernandez de Elche |
July 2015 | Not Applicable |
| NCT01539655 | Completed | Medullary Thyroid Cancer |
Sanofi |
February 2012 | Phase 1 |
| NCT01017198 | Completed | Advanced Solid Tumors |
Biogen |
November 2009 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































