research use only
Chlorpheniramine Maleate Histamine Receptor antagonist
Cat.No.S1816
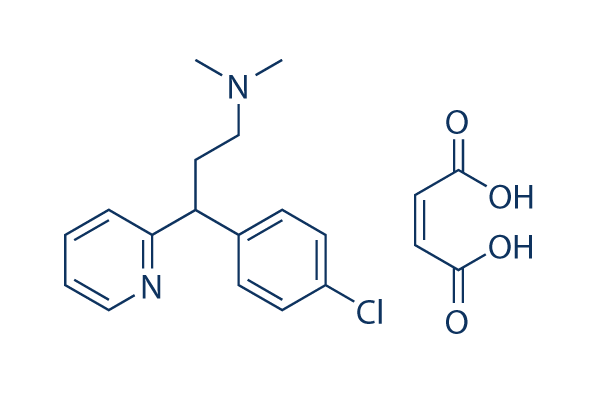
Chemical Structure
Molecular Weight: 390.86
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) GluR |
|---|---|
| Other Histamine Receptor Inhibitors | GSK2879552 Dihydrochloride JNJ-7777120 Ebastine Mianserin HCl Astemizole Ciproxifan Maleate Lafutidine Mizolastine Dimethindene maleate Rupatadine |
Solubility
|
In vitro |
DMSO
: 78 mg/mL
(199.55 mM)
Water : 78 mg/mL Ethanol : 78 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 390.86 | Formula | C16H19ClN2.C4H4O4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 113-92-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | NCI-C55265 | Smiles | CN(C)CCC(C1=CC=C(C=C1)Cl)C2=CC=CC=N2.C(=CC(=O)O)C(=O)O | ||
Mechanism of Action
| Targets/IC50/Ki |
Histamine H1 receptor
12 nM
|
|---|---|
| In vitro |
Chlorpheniramine inhibits the [3H]mepyramine binding to the histamine H1 receptor in guinea pig cortex with IC50 of 8.8 nM. Chlorpheniramine inhibits the proliferation of MCF-7, MDA-MB 231, and Ehrlich cells in a dose-response manner, and significantly reduces the ornithine decarboxylase mRNA translation by 50%-70% at the 250 μM. Chlorpheniramine displaces of [3H]pyrilamine from human histamine receptor subtype 1 expressed in CHO cells with IC50 of 66 nM. Chlorpheniramine displays antimalarial activity against CQS strain (D6) and MDR strain (Dd2) of P. falciparum with IC50 of 61.2 uM and 3.9 uM, respectively. Chlorpheniramine displays cytotoxicity against the proliferation of concanavalin A-induced murine splenic lymphocytes with IC50 of 33.4 μM. Chlorpheniramine treatment in human salivary gland cells more significantly inhibits the histamine-induced [Ca2+]i increase in a concentration-dependent manner with IC50 of 128 nM than that of carbachol-induced [Ca2+]i increase with an IC50 of 43.9 μM.
|
| Kinase Assay |
H1-Antihistaminic Activity
|
|
The segments (1 cm) of isolated ileum from guinea pigs are suspended in an organ bath containing Tyrode solution (ventilation, 32 °C). The contractile responses to histamine (0.54 μM) are measured with an isotonic transducer. A set concentration of Chlorpheniramine is added in the organ bath 5 minutes before the addition of histamine. IC50 value of Chlorpheniramine is calculated by the probit methond.
|
|
| In vivo |
Oral administration of Chlorpheniramine inhibits histamine-induced mortality in guinea pigs with an ED50 of 0.17 mg/kg. Oral administration of Chlorpheniramine (10 mg/kg) significantly inhibits short-duration scratching in BALB/c mice stimulated by ovalbumin active cutaneous anaphylaxis and in ICR mice subcutaneously injected with histamine, but not long-duration scratching seen in NC/Nga mice, in contrast to that of dexamethasone or tacrolimus. Administration of Chlorpheniramine (20 mg/kg) significantly abolishes the increase in REM sleep in rats induced by immobilization stress due to the blockage of the histaminergic and cholinergic mechanisms generating REM sleep.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































