research use only
Bupivacaine HCl Sodium Channel inhibitor
Cat.No.S2454
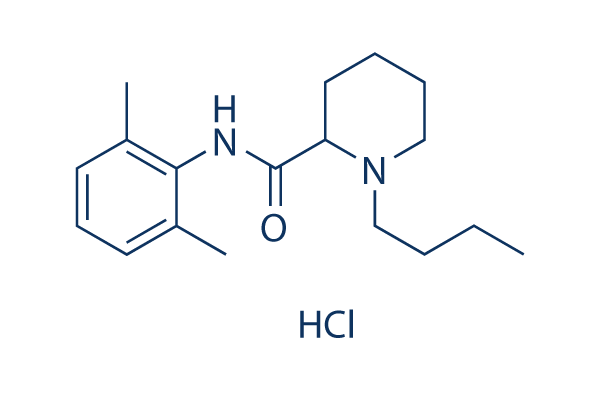
Chemical Structure
Molecular Weight: 324.89
Quality Control
| Related Targets | CFTR CRM1 CD markers AChR Calcium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other Sodium Channel Inhibitors | Camostat Mesilate A-803467 cariporide Veratramine Tolperisone HCl Bulleyaconi cine A Vinpocetine Tenapanor PF-06869206 Sparteine |
Solubility
|
In vitro |
DMSO
: 65 mg/mL
(200.06 mM)
Water : 65 mg/mL Ethanol : 65 mg/mL |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 324.89 | Formula | C18H28N2O.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 18010-40-7 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CCCCN1CCCCC1C(=O)NC2=C(C=CC=C2C)C.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
Sodium channel
|
|---|---|
| In vitro |
Bupivacaine solution is cytotoxic to bovine articular chondrocytes and articular cartilage in vitro after only 15 to 30 minutes exposure. Bupivacaine acts in isolated mitochondria, as uncouplers between oxygen consumption and phosphorylation of adenosine diphosphate. Bupivacaine causes a concentration-dependent mitochondrial depolarization and pyridine nucleotide oxidation in isolated mitochondria, which are matched by an increased oxygen consumption at bupivacaine concentrations of 1.5 mm or less at pH 7.4, whereas respiration is inhibited at higher concentrations. Bupivacaine causes the opening of the permeability transition pore (PTP), a cyclosporin A-sensitive inner membrane channel that plays a key role in many forms of cell death. Bupivacaine causes mitochondrial depolarization and pyridine nucleotides oxidation that are matched by increased concentrations of cytosolic free Ca(2+), release of cytochrome c, and eventually, hypercontracture in intact flexor digitorum brevis fibers. Bupivacaine inhibits GIRK channels within seconds of application, regardless of whether channels are activated through the muscarinic receptor or directly via coexpressed G protein G(beta)gamma subunits. Bupivacaine also inhibits alcohol-induced GIRK currents in the absence of functional pertussis toxin-sensitive G proteins. Bupivacaine HCl also potently inhibits cAMP production with an IC50 of 2.3 μM.
|
| In vivo |
Bupivacaine does not only induce Ca2+ release from the sarcoplasmic reticulum (SR) in rats, but also inhibits Ca2+ uptake by the SR, which is mainly regulated by SR Ca2+ adenosine triphosphatase activity.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05038956 | Not yet recruiting | Video-assisted Thoracic Surgery (VATS) |
University of Utah |
May 1 2024 | -- |
| NCT06252662 | Not yet recruiting | Breast Cancer |
United States Naval Medical Center Portsmouth |
April 2024 | Phase 4 |
| NCT06350981 | Not yet recruiting | Back Pain|Surgery-Complications|Narcotic Use|Physical Stress|Post Operative Pain |
Foundation for Orthopaedic Research and Education|Pacira Pharmaceuticals Inc |
April 1 2024 | Phase 2|Phase 3 |
| NCT04003506 | Not yet recruiting | Pain Postoperative |
The University of Hong Kong |
March 1 2024 | Phase 4 |
| NCT06293404 | Not yet recruiting | Hip Fractures|Spinal Anesthesia |
Ankara City Hospital Bilkent |
March 1 2024 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































