research use only
Sapitinib (AZD8931) HER2 inhibitor
Cat.No.S2192
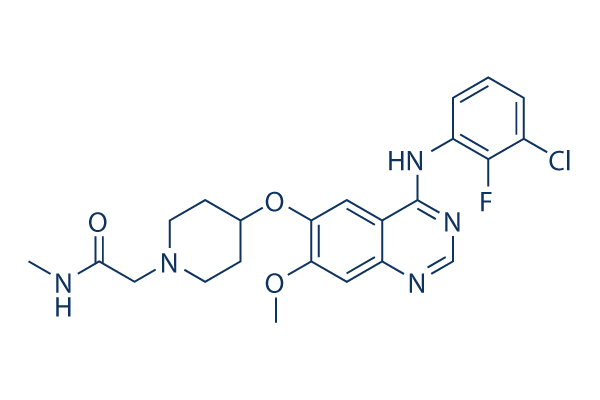
Chemical Structure
Molecular Weight: 473.93
Quality Control
| Related Targets | EGFR VEGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 c-Kit |
|---|---|
| Other HER2 Inhibitors | CP-724714 Mubritinib (TAK 165) AC480 (BMS-599626) Tyrphostin AG 879 HER2-Inhibitor-1 TAS0728 Zongertinib |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| MCF7 | Function assay | Inhibition of heregulin-stimulated HER2 phosphorylation in human MCF7 cells, IC50 = 0.003 μM. | 24900741 | |||
| MCF7 | Function assay | Inhibition of heregulin-stimulated HER3 phosphorylation in human MCF7 cells, IC50 = 0.004 μM. | 24900741 | |||
| KB | Function assay | Inhibition of EGF-stimulated EGFR phosphorylation in human KB cells, IC50 = 0.004 μM. | 24900741 | |||
| MCF-7 | Function assay | 4 hrs | Inhibition of full-length HER2 phosphorylation transfected in in human MCF-7 cl.24 cells after 4 hrs by laser scanning fluorescence microplate cytometry, IC50 = 0.06 μM. | 24900741 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB1643 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SK-N-MC cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for LAN-5 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB-EBc1 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh18 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh30 cells | 29435139 | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 40 mg/mL
(84.4 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 473.93 | Formula | C23H25ClFN5O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 848942-61-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CNC(=O)CN1CCC(CC1)OC2=C(C=C3C(=C2)C(=NC=N3)NC4=C(C(=CC=C4)Cl)F)OC | ||
Mechanism of Action
| Targets/IC50/Ki |
ErbB2
(Cell-free assay) 3 nM
EGFR
(Cell-free assay) 4 nM
ErbB3
(Cell-free assay) 4 nM
|
|---|---|
| In vitro |
Sapitinib (AZD8931) shows different potency to NSCLC and SCCHN cell lines, with high sensitivity to PC-9 cells (EGFR activating mutation) with GI50 of 0.1 nM and low activity to NCI-1437 cells with GI50 above 10 μM. This compound exhibits more potency against phospho-EGFR, phospho-erbB2 and phospho-erbB3 in PE/CA-PJ41, PE/CA-PJ49, DOK and FaDu cells. |
| Kinase Assay |
Isolated kinase assays
|
|
The intracellular kinase domains of human EGFR and erbB2 are cloned and expressed in the baculovirus/Sf21 system. Using the ELISA method, the inhibitory activity of Sapitinib (AZD8931) is determined with ATP at Km concentrations (0.4 mM for erbB2 and 2 mM for EGFR).
|
|
| In vivo |
Sapitinib (AZD8931) reveals antitumor activity in BT474c, Calu-3, LoVo, FaDu and PC-9 xenografts. It could reduce p-Akt, Ki67 expression and p-ERK in BT474c xenografts following acute treatment. This compound also causes induction of the M30 apoptosis marker. Furthermore, it shows greater proapoptotic effect in LoVo xenografts. |
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pEGFR / EGFR / pAKT / AKT / pERK / ERK HER3 / p-HER3 / HER2 / p-HER2 / p-PRAS40 / p-S6 / p-4EBP1 / p-FOXO / PARP / cleaved PARP |
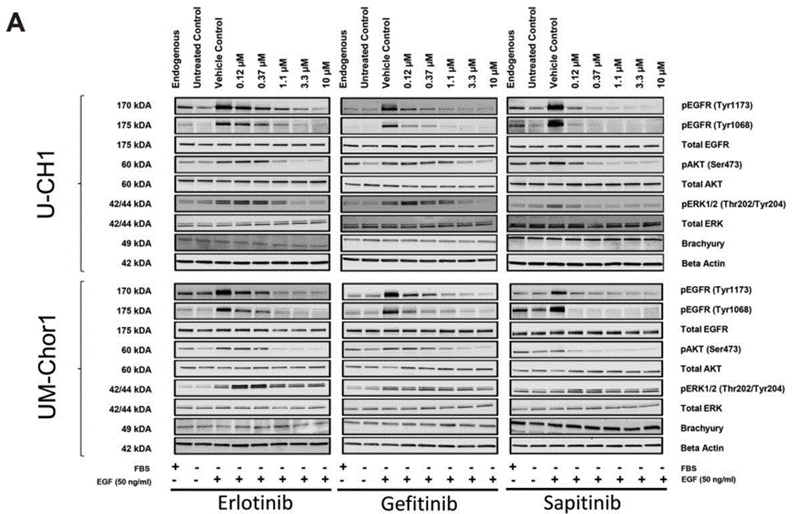
|
27102572 |
| Growth inhibition assay | Cell viability |
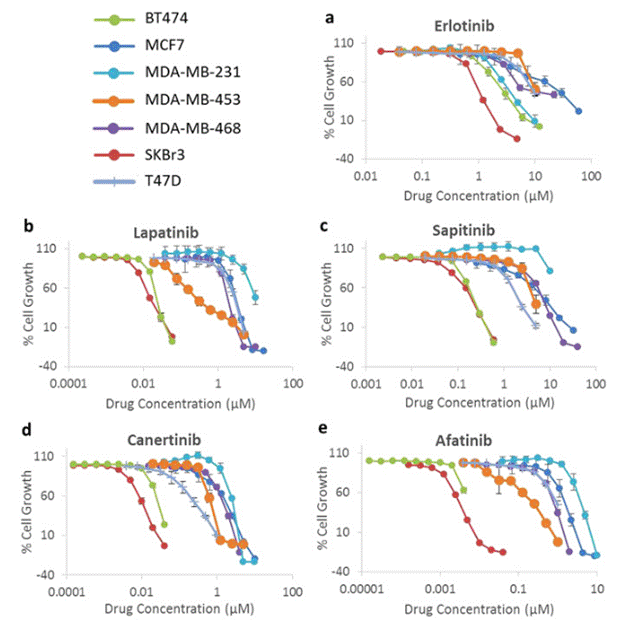
|
28638122 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01596530 | Terminated | Breast Neoplasm |
AstraZeneca |
June 2012 | Phase 1 |
| NCT01579578 | Terminated | Metastatic Gastric or Gastro-oesophageal Junction Cancer |
AstraZeneca |
April 2012 | Phase 2 |
| NCT01330758 | Completed | Healthy |
AstraZeneca |
April 2011 | Phase 1 |
| NCT01284595 | Completed | Healthy |
AstraZeneca |
March 2011 | Phase 1 |
| NCT01151215 | Terminated | Neoplasms|Breast Neoplasms|Breast Cancer |
AstraZeneca |
June 2010 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































