research use only
6-Iodopravadoline (AM630) Cannabinoid Receptor antagonist
Cat.No.S8033
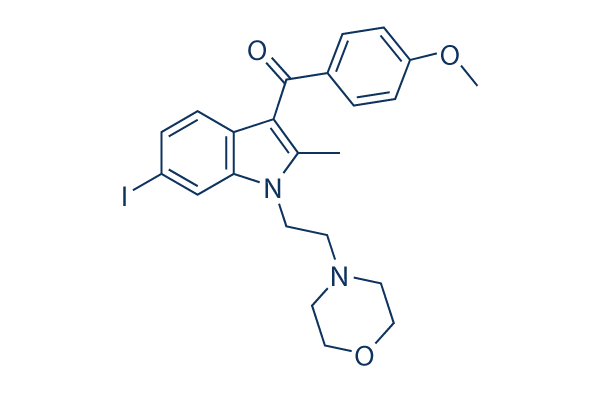
Chemical Structure
Molecular Weight: 504.36
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas |
|---|---|
| Other Cannabinoid Receptor Inhibitors | AM1241 AM251 BML-190 Org 27569 Otenabant (CP-945598) HCl GW842166X CID16020046 WIN 55, 212-2 mesylate Pregnenolone monosulfate sodium RTICBM-189 |
Solubility
|
In vitro |
DMSO
: 50 mg/mL
(99.13 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 504.36 | Formula | C23H25IN2O3 |
Storage (From the date of receipt) | 3 years-21°C powder |
|---|---|---|---|---|---|
| CAS No. | 164178-33-0 | -- | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | COC1=CC=C(C=C1)C(=O)C2=C(C)[N](CCN3CCOCC3)C4=C2C=CC(=C4)I | ||
Mechanism of Action
| Targets/IC50/Ki |
CB2 receptor
(Cell-free assay) 31.2 nM(Ki)
|
|---|---|
| In vitro |
AM630 (6-Iodopravadoline) is a CB2 cannabinoid receptor ligand with Ki of 31.2 nM and a CB2 /CB1 affinity ratio of 165. It inhibits [35 S]-GTPγS binding to CB2 receptor membranes (EC50=76.6 nM), enhances forskolin-stimulated cyclic AMP production in CB2-transfected cells (5.2 fold by 1 μM ), and antagonizes the inhibition of forskolin-stimulated cyclic AMP production in this cell line induced by CP55940. This compound (10 μM) inhibits forskolin-stimulated cyclic AMP production In CB1-transfected cells by 45.9%. It behaves as a competitive antagonist of △9-THC, CP 55,940, WIN 55212-2, anandamide and (R)-(+)-arachidonyl-l’-hydroxy-2’-propylamide (AM356) in mouse isolated vas deferens with Kd of 14.0, 17.3, 36.5, 278.8, and 85.9 nM, respectively.
At 10 μM, it activates a robust Ca2+ accumulation in a subset (35- 40%) of TG neurons, and with EC50 of 15.6 μM. This compound is able to generate currents in TG sensory neurons with the activation threshold of 1 μM. Its responses are mediated by the TRPA1 channel in a majority of TG small-to-medium sensory neurons, which is modulated by TRPV1. Pre-treatment with AM630 (25 μM) is able to inhibits the Capsaicin (CAP) effects, mustard oil (MO) and WIN 55,212-2 (WIN) TRPA1 mediated responses.
At 100 nM, it effectively inhibits osteoclastogenesis in culture with RANKL in the presence and absence of Ti particles, as reducing the number of tartrate-resistant acid phosphatase-positive cells by more than 50%. This compound (100 nM) inhibits mRNA expression of RANK and cathepsin K in RAW cells stimulated by Ti particles and RANKL. It (100 nM) reduces protein expression of interleukin-1β and tumor necrosis factor-α in RAW cells cultured with Ti particles. AM630 has no toxic effect on RAW cells.
|
| In vivo |
6-Iodopravadoline (AM630) (30 μg) injection is not able to induce nociceptive behaviors or thermal hyperalgesia in hind paw of WT mice, but significantly attenuates CAP-induced thermal hyperalgesia. This compound (30 μg) is able to reverse WIN inhibition of CAP-induced thermal hyperalgesia. It exerts its peripheral effects by not only inhibiting CB1 and CB2, but also activating TRPA1 channels and subsequently desensitizing TRPA1 as well as TRPV1 channels.
|
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































