research use only
Tolvaptan Vasopressin Receptor antagonist
Cat.No.S2593
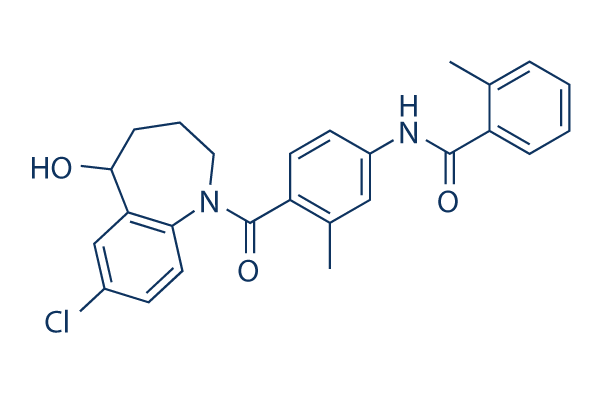
Chemical Structure
Molecular Weight: 448.94
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas |
|---|---|
| Other Vasopressin Receptor Inhibitors | Mozavaptan RO 5028442 (RG7713) Lixivaptan (VPA-985) Mozavaptan (hydrochloride) |
Solubility
|
In vitro |
DMSO
: 90 mg/mL
(200.47 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 448.94 | Formula | C26H25ClN2O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 150683-30-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | OPC-41061 | Smiles | CC1=CC=CC=C1C(=O)NC2=CC(=C(C=C2)C(=O)N3CCCC(C4=C3C=CC(=C4)Cl)O)C | ||
Mechanism of Action
| Targets/IC50/Ki |
vasopressin receptor 2
3 nM
|
|---|---|
| In vitro |
Tolvaptan blocks the binding of [(3)H]AVP to human V(2) receptors with 29-fold greater selectivity than that for V(1a) receptors, and showed no inhibition of V(1b) receptors. This compound inhibits not only the binding of [(3)H]AVP but also the AVP-induced production of cyclic AMP in human V(2)-receptor-expressing HeLa cells. It shows marked aquaresis in healthy and diseased animals. This chemical causes a concentration-dependent inhibition of arginine vasopressin-induced cAMP production with an apparent IC(50) of 0.1 nM in autosomal dominant polycystic kidney disease (ADPKD) cells. It inhibits AVP-induced ERK signaling and cell proliferation. This compound also inhibits AVP-induced Cl(-) secretion and decreases in vitro cyst growth of ADPKD cells cultured within a three-dimensional collagen matrix.
|
| In vivo |
Tolvaptan improves hyponatremia, resulting in the prevention of death, and improves organ water retention in rat models with acute and chronic hyponatremia. This compound reduces cardiac preload without unfavorable effects on renal functions, systemic hemodynamics, or circulating neurohormones in dogs with heart failure (HF). It shows a decrease in kidney weight as well as in cyst and fibrosis volume in animal models of human polycystic kidney disease (PKD). This agent markedly elevates electrolyte-free water clearance (E-CH(2)O) or aquaresis to a positive value and increases urinary Arginine vasopressin (AVP) excretion in rats with heart failure.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06171100 | Recruiting | Hyponatremia|SIADH |
King''s College Hospital NHS Trust |
March 15 2024 | -- |
| NCT04182958 | Completed | Healthy Adult Male |
Otsuka Pharmaceutical Co. Ltd. |
November 25 2019 | Phase 1 |
| NCT02994394 | Completed | Healthy Adult Male |
Otsuka Pharmaceutical Co. Ltd. |
January 6 2017 | Phase 1 |
| NCT02729662 | Unknown status | Autosomal Dominant Polycystic Kidney Disease |
Kyorin University |
October 1 2016 | Not Applicable |
| NCT02020278 | Terminated | Hyponatremia |
Otsuka Pharmaceutical Development & Commercialization Inc.|Syneos Health |
April 22 2016 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































