research use only
Sevelamer HCl FXR inhibitor
Cat.No.S4129
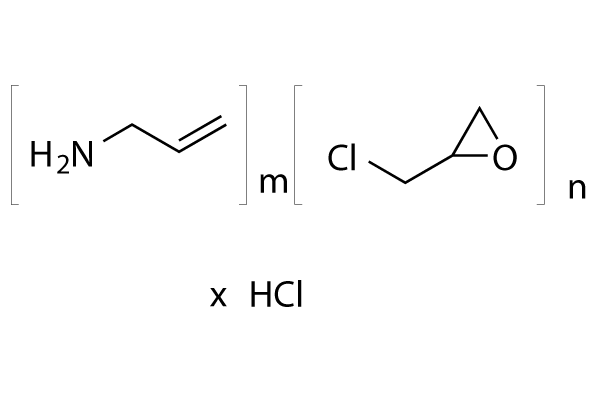
Chemical Structure
Quality Control
Solubility
|
In vitro |
4-Methylpyridine : 5 mg/mL
DMSO
: Insoluble
Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | Formula | (C3H7N.C3H5ClO.HCl)x |
Storage (From the date of receipt) | ||
|---|---|---|---|---|---|
| CAS No. | 152751-57-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C=CCN.C1C(O1)CCl.Cl | ||
Mechanism of Action
| In vivo |
Sevelamer is as effective as CaCO3 in reducing serum phosphorus, calcium-phosphorus product, and attenuating secondary hyperparathyroidism in nephrectomized rats (U) fed high phosphorus (HP) diet. Sevelamer results in markedly lower calcium deposition in the myocardium and aorta compared to control rats. Sevelamer suppresses calcification of the aorta media, and also the osteoid volume, fibrosis volume, and porosity ratio of femurs in chronic renal failure rats. Sevelamer results in a significantly lower degree of atherosclerosis and vascular calcification in uremic mice when compared with uremic control mice. Sevelamer exerts an effect on both intima and media calcification in uremic mice. Sevelamer treatment controlled serum P independent of increases in serum Ca, thus reducing serum calcium-phosphate product and further deterioration of renal function, as indicated by the highest creatinine clearances in uremic rats. Sevelamer is as effective as CaCO3 in the control of high-P-induced SH, as shown by similar serum PTH levels, parathyroid (PT) gland weight, and markers of PT hyperplasia. Sevelamer causes a dramatic reduction of renal Ca deposition compared with both uremic + high-P diet (U-HP) and the U-HP+CaCO3 diet. Sevelamer hydrochloride results in a fall in urine pH, as well as an increase in urinary ammonium and calcium excretion consistent with an increase in net acid excretion in animal model.
|
References |
|
|---|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT01220843 | Completed | Chronic Renal Failure |
Centre Hospitalier Universitaire Amiens|Genzyme a Sanofi Company |
October 2010 | Phase 3 |
| NCT01191762 | Completed | Hyperparathyroidism|Chronic Kidney Disease |
Kenneth R. Phelps M.D.|Genzyme a Sanofi Company|Phelps Kenneth R. M.D. |
April 2010 | Phase 3 |
| NCT01011699 | Terminated | Chronic Renal Failure|Hemodialysis |
Centre Hospitalier Universitaire Amiens |
January 2010 | Phase 3 |
| NCT00853242 | Completed | Kidney Failure Chronic |
Genzyme a Sanofi Company|Sanofi |
February 2009 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































