research use only
MNS (3,4-Methylenedioxy-β-nitrostyrene) p97 inhibitor
Cat.No.S4921
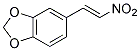
Chemical Structure
Molecular Weight: 193.16
Quality Control
| Related Targets | Proteasome E1 Activating E3 Ligase DUB SUMO E2 conjugating |
|---|---|
| Other p97 Inhibitors | NMS-873 DBeQ CB-5339 NPD8733 ML240 |
Solubility
|
In vitro |
DMSO
: 39 mg/mL
(201.9 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 193.16 | Formula | C9H7NO4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1485-00-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | MDBN | Smiles | C1OC2=C(O1)C=C(C=C2)C=C[N+](=O)[O-] | ||
Mechanism of Action
| Features |
The nitro group of MNS (3,4-Methylenedioxy-β-nitrostyrene) is essential for its antiplatelet effect.
|
|---|---|
| Targets/IC50/Ki |
p97
1.7 μM
Syk
2.5 μM
Src
29.3 μM
|
| In vitro |
MNS (3,4-methylenedioxy-β-nitrostyrene) completely inhibits 2 μM U46619-(a thromboxane A2 mimic), 5 μM ADP-, 100 μM arachidonic acid-(AA), 10 μg/ml collagen-, and 0.1 U/ml thrombin-induced platelet aggregation in a concentration-dependent manner with IC50 of 2.1 μM, 4.1 μM, 5.8 μM, 7.0 μM, and 12.7 μM, respectively. It inhibits platelet aggregation caused by either the calcium ionophore A23187 (1 μM) or the protein kinase C (PKC) activator PDBu (200 nM) with IC50 of 25.9 μM and 4.8 μM, respectively. This compound (20 μM) decreases thrombin-induced P-selectin expression on platelets to levels comparable to those observed in PGE1-treated platelets. It (20 μM) markedly inhibits thrombin-but not PDBu-induced MARCKS phosphorylation in platelets. MNS (20 μM) markedly inhibits protein tyrosine phosphorylation at either 0.5 min or 3 min after thrombin or collagen stimulation in platelets. It stimulates UbG76V-GFP and ODD-Luc degradation with IC50 of 1.6 μM and 5.9 μM, respectively, and inhibits MG132-induced accumulation of the reporter with IC50 of 2.1 μM. MNS inhibits Gram-positive (Staphylococcus aureus and Enterococcus faecalis) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria with minimum inhibitory concentrations (MICs) of 128 mg/L. It is much more potent than genistein in inhibiting platelet aggregation and protein tyrosine phosphorylation, and is equally potent as inhibitors of platelet aggregation as 3,4-dimethoxy-β-nitrostyrene. This compound (20 μM) concentration-dependently prevents ATP release from platelets stimulated by thrombin or collagen, and inhibits thrombin-induced PAC-1 binding to human platelets.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04473027 | Terminated | Melanoma|Cancer |
University Hospital Ghent|Jessa Hospital|University Hospital Antwerp|Algemeen Ziekenhuis Maria Middelares|GZA Ziekenhuizen Campus Sint-Augustinus|AZ Sint-Jan AV|AZ Nikolaas|AZ Sint-Lucas Gent|AZ Sint-Lucas Brugge|General Hospital Groeninge|OLV van Lourdes Hospital Waregem|AZ Damiaan|AZ Delta|ASZ Aalst|Belgian Red Cross |
September 1 2020 | -- |
| NCT01362244 | Completed | Nasal Polyps |
GlaxoSmithKline |
May 12 2009 | Phase 2 |
| NCT00474799 | Completed | Healthy |
Javelin Pharmaceuticals |
January 2007 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































