research use only
Eltanexor (KPT-8602) CRM1 inhibitor
Cat.No.S8397
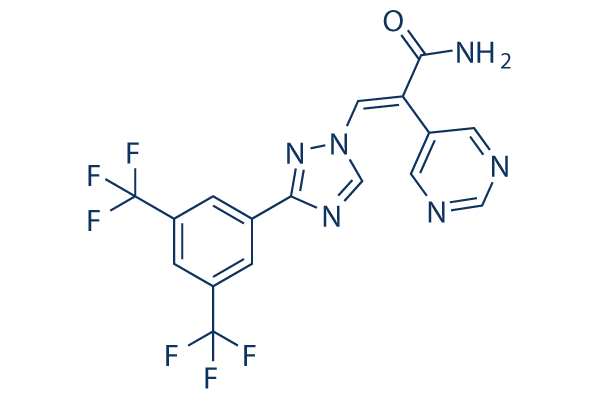
Chemical Structure
Molecular Weight: 428.29
Quality Control
| Related Targets | CFTR CD markers AChR Calcium Channel Sodium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other CRM1 Inhibitors | KPT-185 Verdinexor (KPT-335) KPT-276 Leptomycin B (LMB) |
Solubility
|
In vitro |
DMSO
: 85 mg/mL
(198.46 mM)
Ethanol : 1 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 428.29 | Formula | C17H10F6N6O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1642300-52-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | ONO-7706,ATG-016 | Smiles | C1=C(C=C(C=C1C(F)(F)F)C(F)(F)F)C2=NN(C=N2)C=C(C3=CN=CN=C3)C(=O)N | ||
Mechanism of Action
| Targets/IC50/Ki |
XPO1
|
|---|---|
| In vitro |
Eltanexor (KPT-8602) is a potent inhibitor of AML cells in cell-based viability assays. It inhibits XPO1/cargo interactions and nuclear export, induces apoptosis of primary CLL cells and significantly inhibits proliferation of diffuse large B-cell lymphoma cell lines
|
| In vivo |
Eltanexor (KPT-8602) is orally bioavailable and has similar pharmacokinetic properties to selinexor, but exhibits markedly reduced (approximately 30-fold less) penetration across the blood−brain barrier. Toxicology studies in rats and monkeys indicate that this compound has a substantially better tolerability profile, probably due to its inability to penetrate into the CNS, with reduced anorexia, malaise and weight loss compared to selinexor. It also shows superior anti-leukemic activity and better tolerability in the AML PDX models tested, with nearly complete elimination of human AML cells in the AML-CN model. It is minimally toxic to normal hematopoietic stem and progenitor cells. Furthermore, it does not accumulate in plasma after repetitive dosing and prolongs survival in a human leukemia xenograft model of AML.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | XPO1 |
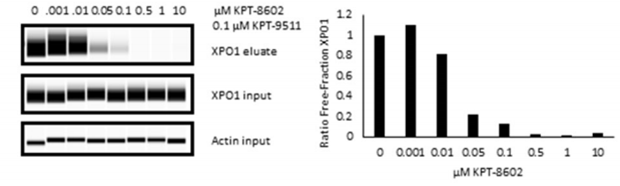
|
26654943 |
| Immunofluorescence | p53 / NPM1 p62 / p53 |
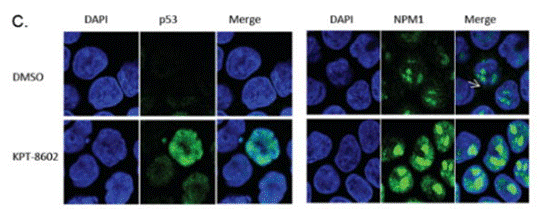
|
27323910 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05918055 | Recruiting | Myelodysplastic Syndromes |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
November 14 2023 | Phase 1|Phase 2 |
| NCT02649790 | Active not recruiting | Relapsed/Refractory Multiple Myeloma (RRMM)|Metastatic Colorectal Cancer (mCRC)|Metastatic Castration-Resistant Prostate Cancer (mCRPC)|Higher-Risk Myelodysplastic Syndrome (HR-MDS)|Acute Myeloid Leukemia (AML)|Newly Diagnosed Intermediate/High-Risk MDS |
Karyopharm Therapeutics Inc |
January 2016 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































