- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Benznidazole Parasite inhibitor
Cat.No.S3741
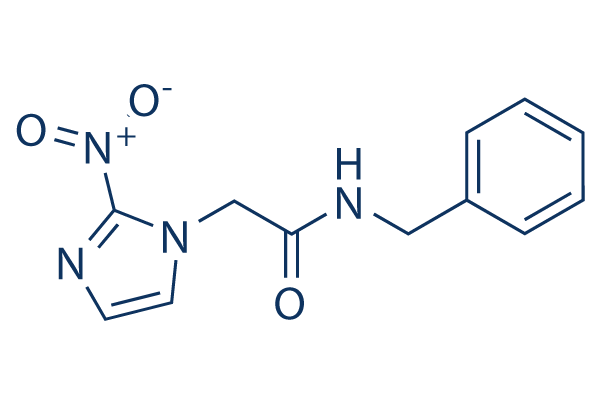
Chemical Structure
Molecular Weight: 260.25
Jump to
Quality Control
Batch:
Purity:
99.87%
99.87
Solubility
|
In vitro |
DMSO
: 52 mg/mL
(199.8 mM)
Ethanol : 5 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 260.25 | Formula | C12H12N4O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 22994-85-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Radanil | Smiles | C1=CC=C(C=C1)CNC(=O)CN2C=CN=C2[N+](=O)[O-] | ||
Mechanism of Action
| In vitro |
Benznidazole (BZL) inhibits the proliferation of leukemic non-adherent cells by controlling cell cycle at G0/G1 cell phase through up-regulation of p27. Growth inhibition induced by this compound is a reversible process, not accompanied by significant cell death. Besides its trypanocidal activity, it also has an immunomodulatory effect on macrophages by blocking the transcription of some pro-inflammatory mediators without altering interleukin 10 expression.
|
|---|---|
| In vivo |
In mice, oral administration of Benznidazole (100 mg/kg): the time to reach maximum concentration (Tmax) in plasma was 0.83 h, and the maximum concentration (Cmax) in plasma was 41.61 μg/ml. The elimination half-life (t1/2b) of this compound was 2.03 h, and mean residence time (MRT) was 3.86 h. The volume of distribution (V) and clearance (CL), both as a function of its bioavailability (F), were 38.81 ml and 13.29 ml/h, respectively. In Wistar rats treated orally, Tmaxs of this chemical are 2.0 and 1.1 h, respectively. Tmaxs of 15, 30, or 60 min, depending on the dose, in BALB/c mice following intraperitoneal treatment and Tmaxs of 1 to 5 h for dogs treated orally. It can cross the blood-brain barrier and exert its action in cases of central nervous system parasitism. However, other studies have indicated that this compound has toxic effects in the central nervous system. Dogs orally treated with it presented encephalopathy with multifocal characteristics and clinical, pathological, and neurological disorders that were dose dependent and time dependent. Its biodistribution occurs broadly, reaching the heart and colon, which are the most relevant organs for T. cruzi infection, and also the spleen, brain, liver, lungs, and kidneys.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03892213 | Completed | Chagas Disease |
Drugs for Neglected Diseases|PhinC Development |
October 2014 | Phase 1 |
| NCT01755403 | Completed | Chagas Disease |
Barcelona Centre for International Health Research |
December 2012 | Phase 4 |
| NCT01547533 | Completed | Chagas Disease|Lactation |
Hospital de Niños R. Gutierrez de Buenos Aires |
August 2011 | -- |
| NCT01489228 | Unknown status | Chronic Chagas Disease Indeterminate |
Drugs for Neglected Diseases|Eisai Co. Ltd. |
June 2011 | Phase 2 |
| NCT00699387 | Completed | Chagas Disease |
Hospital de Niños R. Gutierrez de Buenos Aires|Thrasher Research Fund|The Hospital for Sick Children|Fundacion Bunge y Born (Argentina)|Universidad Nacional de La Plata|Consejo de Investigacion en Salud Gobierno de Buenos Aires |
April 2007 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































