- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Lanabecestat (AZD3293) BACE inhibitor
Cat.No.S8193
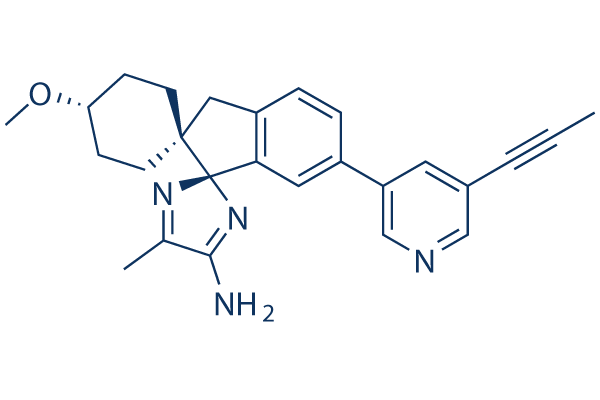
Chemical Structure
Molecular Weight: 412.53
Jump to
Quality Control
Batch:
Purity:
99.97%
99.97
Solubility
|
In vitro |
DMSO
: 82 mg/mL
(198.77 mM)
Ethanol : 82 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 412.53 | Formula | C26H28N4O |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1383982-64-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LY3314814 | Smiles | CC#CC1=CC(=CN=C1)C2=CC3=C(CC4(C35N=C(C(=N5)N)C)CCC(CC4)OC)C=C2 | ||
Mechanism of Action
| Targets/IC50/Ki |
BACE
(Cell-free assay) 0.4 nM(Ki)
|
|---|---|
| In vitro |
Lanabecestat (AZD3293, LY3314814) is a potent, highly permeable, orally active, blood-brain barrier (BBB) penetrating, BACE1 inhibitor with unique slow off-rate kinetics. When the potency of this compound with respect to secretion of Aβ40 and sAβPPβ is studied in a range of cellular models, it displays pM potency in primary neuron cultures from mice and guinea pigs and in SH-SY5Y cells over-expressing AβPP (IC50 = 610 pM, 310 pM, and 80 pM, respectively). It is also tested in a panel of more than 350 in vitro radioligand binding and enzyme activity assays, covering a diverse range of receptors, ion channels, transporters, kinases, and enzymes, up to a concentration of 10μM. A few significant responses are observed, but these had at least a 1,000-fold selectivity against BACE1, thus indicating specificity to BACE1. The off-rate of AZD3293 has an estimated t1/2 of approximately 9 h.
|
| In vivo |
Lanabecestat (AZD3293) displays significant dose- and time-dependent reductions in plasma, cerebrospinal fluid, and brain concentrations of Aβ40, Aβ42, and sAβPPβ in vivo in mice, guinea pigs, and dogs. In the dog PK study, its bioavailability is determined to be 80% (F = 0.8). The preclinical data strongly support the clinical development of this compound, and patients with AD are currently being recruited into a combined Phase 2/3 study to test its disease-modifying properties.
|
References |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03499041 | Withdrawn | Hepatic Impairment |
Eli Lilly and Company|AstraZeneca |
June 2018 | Phase 1 |
| NCT03222427 | Completed | Healthy |
AstraZeneca|Eli Lilly and Company |
January 15 2018 | Phase 1 |
| NCT03019549 | Completed | Healthy |
AstraZeneca|Eli Lilly and Company |
January 12 2017 | Phase 1 |
| NCT02663128 | Completed | Healthy |
AstraZeneca|Eli Lilly and Company |
January 31 2016 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































