- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Ramelteon MT Receptor agonist
Cat.No.S1259
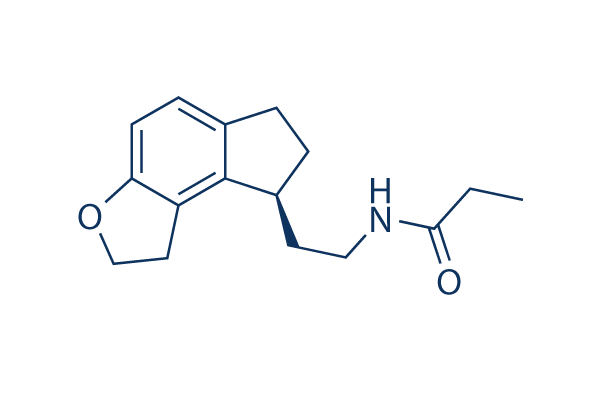
Chemical Structure
Molecular Weight: 259.34
Jump to
Quality Control
Batch:
Purity:
99.92%
99.92
| Related Targets | K-Ras CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras |
|---|---|
| Other MT Receptor Products | Melatonin Luzindole |
Solubility
|
In vitro |
DMSO
: 51 mg/mL
(196.65 mM)
Ethanol : 51 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 259.34 | Formula | C16H21NO2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 196597-26-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | TAK-375 | Smiles | CCC(=O)NCCC1CCC2=C1C3=C(C=C2)OCC3 | ||
Mechanism of Action
| Features |
A tricyclic synthetic analog of melatonin.
|
|---|---|
| Targets/IC50/Ki |
MT1 receptor
(Cell-free assay) 14 pM(Ki)
MT receptor (chicken)
(Cell-free assay) 23.1 pM(Ki)
MT2 receptor
(Cell-free assay) 112 pM(Ki)
|
| In vitro |
Ramelteon inhibits forskolin-stimulated cAMP production with IC50 of 21.2 pM in CHO cells. This compound has high affinity with recombinant human MT1 and MT2 receptors with pKi of 10.05 and 9.70, respectively. It inhibits Xenopus laevis melanophore pigment granule aggregation with pEC50 of 11.48. This chemical (1 nM) increases ERK1/2 phosphorylation not only in MT1/MT2 cerebellar granule cells but also in cerebellar granule cells expressing only one of the two melatonin receptors. 4P-PDOT blocks the stimulatory action of this compound (1 nM) in MT1 KO cerebellar granule cells, while luzindole attenuates the action of this chemical (1 nM) in MT2 KO cerebellar granule cells. It (100 μM) induces any pigment dispersion while melatonin completely disperses aggregated melanophores at 10 μM.
|
| In vivo |
Ramelteon (10 mg/kg, i/p) significantly reduces NREM sleep latency in rat and also produces a short-lasting increase in nonrapid eye movement (NREM) sleep duration, but the NREM power spectrum is unaltered. This compound (0.1 mg/kg and 1 mg/kg, p.o.) accelerates reentrainment of running wheel activity rhythm to the new lightdark cycle in rats without affecting learning or memory. It (0.03 mg/kg and 0.3 mg/kg, p.o.) significantly shortens latency to sleep onset and significantly increases total duration of sleep in freely moving monkeys without affecting the general behavior of the monkeys.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03931070 | Completed | Delirium |
Rhode Island Hospital |
May 9 2019 | Phase 4 |
| NCT02560324 | Completed | Tobacco Use Disorder |
University of Pennsylvania|National Institute on Drug Abuse (NIDA)|National Institutes of Health (NIH) |
September 2015 | Phase 2 |
| NCT02969343 | Completed | Central Line-Associated Bloodstream Infection (CLABSI)|Venous Thromboembolism|Patient Fall|Catheter-Associated Infection|Severe Hypoglycemia|Opioid-Related Severe Adverse Drug Event|Hospital Acquired Pressure Ulcer|Adverse Drug Event|Severe Hospital Acquired Delerium|Rapid Response Related to Arrhythmia |
Brigham and Women''s Hospital|Northeastern University |
April 2015 | Not Applicable |
| NCT00568789 | Completed | Insomnia |
Takeda |
June 2006 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































