research use only
Ataluren (PTC124) CFTR inhibitor
Cat.No.S6003
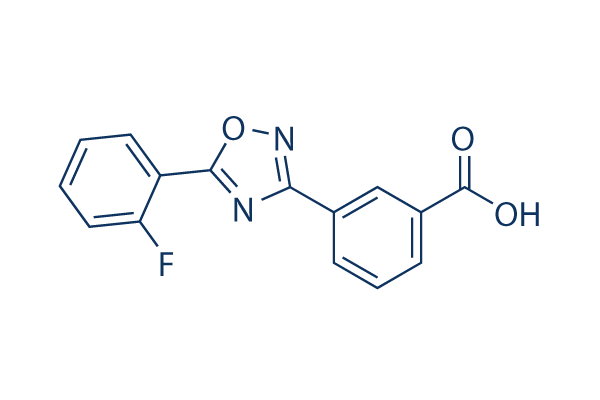
Chemical Structure
Molecular Weight: 284.24
Quality Control
| Related Targets | CRM1 CD markers AChR Calcium Channel Sodium Channel Potassium Channel GABA Receptor TRP Channel ATPase GluR |
|---|---|
| Other CFTR Inhibitors | Tezacaftor (VX-661) VX-445 (Elexacaftor) Vanzacaftor (VX-121) CFTRinh-172 GLPG1837 FDL169 Galicaftor (ABBV-2222) GlyH-101 IOWH032 PPQ-102 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| BAC_MMA∗EGFP | Function Assay | 20 μM | 72 h | DMSO | results in a significant increase in median peak fluorescence | |
| Mut−/−MUTStop+/− | Function Assay | 20 μM | 72 h | DMSO | increases the amount of MUT mRNA | |
| HEK293T | Function Assay | 10 µg/ml | DMSO | restores full-length harmonin a1 (∼80 kDa) in p.R31X-transfected cells | ||
| ML1 | Function Assay | 3.3/10 μM | 48 h | DMSO | increases ARSB activity | |
| ML2 | Function Assay | 3.3/10 μM | 48 h | DMSO | increases ARSB activity | |
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 57 mg/mL
(200.53 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 284.24 | Formula | C15H9FN2O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 775304-57-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | C1=CC=C(C(=C1)C2=NC(=NO2)C3=CC(=CC=C3)C(=O)O)F | ||
Mechanism of Action
| Features |
Demonstrates oral bioavailability, and an appropriate safety toxicology profile.
|
|---|---|
| Targets/IC50/Ki |
CFTR (nonsense mutant)
(HEK293 cells) |
| In vitro |
Ataluren (PTC124) is a more potent nonsense-suppressing agent and exhibits 4- to 15-fold stimulation of read-through relative to controls. This compound (0.01-3 μM) promotes dose-dependent read-through of all three nonsense codons in HEK293 cells harboring LUC-190 nonsense alleles with the highest read-through at UGA, followed by UAG and then UAA, but it does not suppress multiple proximal nonsense codons. It is most active when a pyrimidine (in particular cytosine, C) follows the nonsense codon. Consistent with the stable cell line reporter assay, PTC124 (17 μM) promotes significant production of dystrophin in primary muscle cells from Duchenne muscular dystrophy (DMD) patients or MDXMDX mice expressing dystrophin nonsense alleles. It selectively promotes ribosomal read-through of premature termination but not normal termination codons, even at concentrations substantially greater than the values achieving maximal activity. |
| In vivo |
Due to functional recovery of dystrophin production, oral, intraperitoneal or combined dosing of Ataluren (PTC124) for 2-8 weeks partially rescues functional strength deficit in dystrophic muscles of MDX mice, and results in partial protection against contraction-induced injury in the extensor digitorum longus (EDL) muscles, as well as significant reductions in serum creatine kinase values. In Cftr-/- mice expressing a human CFTR-G542X transgene, subcutaneous or oral administration of this compound (~60 mg/kg) suppresses the G542X nonsense mutation in a dose-dependent manner, leading to a significant restoration of human (h)CFTR protein expression and function without any effect on nonsense-mediated mRNA decay (NMD) or other aspects of mRNA stability. Treatment with it (60 mg/kg) restores 29% of the normal intestinal transepithelial cAMP-stimulated shortcircuit currents observed in Cftr+/+ mice, displaying a significant advantage. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04336826 | Completed | Nonsene Mutation Duchenne Muscular Dystrophy |
PTC Therapeutics |
December 29 2021 | Phase 2 |
| NCT04014530 | Recruiting | Colorectal Cancer|Endometrium Cancer |
Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Merck Sharp & Dohme LLC|PTC Therapeutics |
August 1 2019 | Phase 1|Phase 2 |
| NCT02758626 | Completed | Epilepsy |
NYU Langone Health|PTC Therapeutics |
November 2016 | Phase 2 |
| NCT02819557 | Completed | Duchenne Muscular Dystrophy |
PTC Therapeutics |
June 9 2016 | Phase 2 |
| NCT02090959 | Terminated | Muscular Dystrophy Duchenne|Muscular Dystrophies|Muscular Disorders Atrophic|Muscular Diseases|Musculoskeletal Diseases|Neuromuscular Diseases|Nervous System Diseases|Genetic Diseases X-Linked|Genetic Diseases Inborn |
PTC Therapeutics |
March 20 2014 | Phase 3 |
| NCT01140451 | Completed | Cystic Fibrosis |
PTC Therapeutics|Cystic Fibrosis Foundation |
August 12 2010 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































