- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Neratinib (HKI-272) HER2/EGFR Inhibitor
Cat.No.S2150
Chemical Structure
Molecular Weight: 557.04
Jump to
Quality Control
Batch:
Purity:
99.94%
99.94
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| BT-474 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| EFM-192A | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| HCC1569 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| HCC1954 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| MDA-MB-175 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| MDA-MB-361 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| SK-BR-3 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| UACC-812 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| UACC-893 | Growth Inhibition Assay | IC50<0.005 μM | 24009064 | |||
| SUM-225 | Growth Inhibition Assay | IC50=0.01 μM | 24009064 | |||
| SUM-190 | Growth Inhibition Assay | IC50=0.01 μM | 24009064 | |||
| ZR-75-1 | Growth Inhibition Assay | IC50=0.03 μM | 24009064 | |||
| HCC70 | Growth Inhibition Assay | IC50=0.03 μM | 24009064 | |||
| BT-20 | Growth Inhibition Assay | IC50=0.07 μM | 24009064 | |||
| MDA-MB-453 | Growth Inhibition Assay | IC50=0.09 μM | 24009064 | |||
| HCC1187 | Growth Inhibition Assay | IC50=0.10 μM | 24009064 | |||
| EFM-19 | Growth Inhibition Assay | IC50=0.11 μM | 24009064 | |||
| T-47D | Growth Inhibition Assay | IC50=0.16 μM | 24009064 | |||
| MDA-MB-134 | Growth Inhibition Assay | IC50=0.17 μM | 24009064 | |||
| HCC38 | Growth Inhibition Assay | IC50=0.25 μM | 24009064 | |||
| MDA-MB-435 | Growth Inhibition Assay | IC50=0.33 μM | 24009064 | |||
| MDA-MB-468 | Growth Inhibition Assay | IC50=0.33 μM | 24009064 | |||
| CAMA-1 | Growth Inhibition Assay | IC50=0.37 μM | 24009064 | |||
| MDA-MB-436 | Growth Inhibition Assay | IC50=0.41 μM | 24009064 | |||
| MCF-7 | Growth Inhibition Assay | IC50=0.41 μM | 24009064 | |||
| MDA-MB-415 | Growth Inhibition Assay | IC50=0.42 μM | 24009064 | |||
| HCC1806 | Growth Inhibition Assay | IC50=0.44 μM | 24009064 | |||
| HCC1395 | Growth Inhibition Assay | IC50=0.49 μM | 24009064 | |||
| HCC1937 | Growth Inhibition Assay | IC50=0.50 μM | 24009064 | |||
| HCC1143 | Growth Inhibition Assay | IC50=0.54 μM | 24009064 | |||
| UACC-732 | Growth Inhibition Assay | IC50=0.65 μM | 24009064 | |||
| MDA-MB-231 | Growth Inhibition Assay | IC50=1.00 μM | 24009064 | |||
| MDA-MB-157 | Growth Inhibition Assay | IC50=1.12 μM | 24009064 | |||
| BT-549 | Growth Inhibition Assay | IC50=1.14 μM | 24009064 | |||
| KPL-1 | Growth Inhibition Assay | IC50=1.89 μM | 24009064 | |||
| CAL-51 | Growth Inhibition Assay | IC50=1.89 μM | 24009064 | |||
| BT474 | Growth Inhibition Assay | IC50=0.00323 ± 0.00075 μM | 23816254 | |||
| SKBR3 | Growth Inhibition Assay | IC50=0.0075 ± 0.005 μM | 23816254 | |||
| MDAMB453 | Growth Inhibition Assay | IC50=1.59 ± 0.179 μM | 23816254 | |||
| KB | Growth Inhibition Assay | IC50=4.13 ± 0.47 μM | 22491935 | |||
| KBv200 | Growth Inhibition Assay | IC50=6.03 ± 0.64 μM | 22491935 | |||
| MCF-7 | Growth Inhibition Assay | IC50=3.30 ± 0.41 μM | 22491935 | |||
| MCF-7/Adr | Growth Inhibition Assay | IC50= 2.88 ± 0.30 μM | 22491935 | |||
| MCF-7 | Growth Inhibition Assay | IC50=3.02 ± 0.34 μM | 22491935 | |||
| MCF-7/FLV1000 | Growth Inhibition Assay | IC50=7.09 ± 0.71 μM | 22491935 | |||
| HL60 | Growth Inhibition Assay | IC50=2.26 ± 0.23 μM | 22491935 | |||
| HL60/Adr | Growth Inhibition Assay | IC50=1.42 ± 0.15 μM | 22491935 | |||
| HEK293/pcDNA3.1 | Growth Inhibition Assay | IC50=5.29 ± 0.53 μM | 22491935 | |||
| HEK293/ABCB1 | Growth Inhibition Assay | IC50=6.91 ± 0.70 μM | 22491935 | |||
| SKBR | Growth Inhibition Assay | 0.01-100 nM | 3-7 d | inhibits cell growth in time and dose dependent manner | 21487605 | |
| L858R(EGFR) | Cell Viability Assay | decreases cell viability in time and dose dependent manner | 17311002 | |||
| L858R/T790M(EGFR) | Cell Viability Assay | decreases cell viability in time and dose dependent manner | 17311002 | |||
| G776insV_G/C | Cell Viability Assay | decreases cell viability in time and dose dependent manner | 17311002 | |||
| wild-type | Cell Viability Assay | decreases cell viability in time and dose dependent manner | 17311002 | |||
| A775insYVMA | Cell Viability Assay | decreases cell viability in time and dose dependent manner | 17311002 | |||
| G776insV_G/L | Cell Viability Assay | decreases cell viability in time and dose dependent manner | 17311002 | |||
| P780insGSP | Cell Viability Assay | decreases cell viability in time and dose dependent manner | 17311002 | |||
| NCI-H1781 | Growth Inhibition Assay | inhibits cell growth in time and dose dependent manner | 16818618 | |||
| HCC827 | Growth Inhibition Assay | inhibits cell growth in time and dose dependent manner | 16818618 | |||
| H3255 | Growth Inhibition Assay | inhibits cell growth in time and dose dependent manner | 16818618 | |||
| NCI-H1975 | Growth Inhibition Assay | inhibits cell growth in time and dose dependent manner | 16818618 | |||
| A549 | Growth Inhibition Assay | inhibits cell growth in time and dose dependent manner | 16818618 | |||
| 3T3 | Growth Inhibition Assay | IC50=700 ± 78 nM | 15173008 | |||
| 3T3/neu | Growth Inhibition Assay | IC50=3 ± 0.14 nM | 15173008 | |||
| SK-Br-3 | Growth Inhibition Assay | IC50=2 ± 0.18 nM | 15173008 | |||
| BT 474 | Growth Inhibition Assay | IC50=2 ± 0.06 nM | 15173008 | |||
| A431 | Growth Inhibition Assay | IC50=81 ± 9 nM | 15173008 | |||
| MDA-MB-435 | Growth Inhibition Assay | IC50=960 ± 165 nM | 15173008 | |||
| SW620 | Growth Inhibition Assay | IC50=690 ± 84 nM | 15173008 | |||
| SKBR3 | Function assay | Inhibition of human Her2 in SKBR3 cells, EC50 = 0.002 μM. | 18077425 | |||
| BT474 | Function assay | Inhibition of human Her2 in BT474 cells, EC50 = 0.002 μM. | 18077425 | |||
| A431 | Function assay | Inhibition of human Her2 in A431 cells, EC50 = 0.081 μM. | 18077425 | |||
| SW620 | Function assay | Inhibition of human Her2 in SW620 cells, EC50 = 0.69 μM. | 18077425 | |||
| BA/F3 | Cytotoxicity assay | Cytotoxicity against mouse BA/F3 cells expressing EGFR L858R mutant, IC50 = 0.0035 μM. | 19239229 | |||
| BA/F3 | Cytotoxicity assay | Cytotoxicity against mouse BA/F3 cells expressing EGFR L858R/T790M mutant, IC50 = 0.18 μM. | 19239229 | |||
| Sf9 | Function assay | 10 mins | Inhibition of human wild type EGFR expressed in Sf9 cells using [gamma32P]-ATP after 10 mins by scintillation counting, IC50 = 0.0025 μM. | 24900643 | ||
| Sf9 | Function assay | 10 mins | Inhibition of human EGFR T790M/L858R mutant expressed in Sf9 cells using [gamma32P]-ATP after 10 mins by scintillation counting, IC50 = 0.066 μM. | 24900643 | ||
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused IGF1R (unknown origin) expressed in mouse BAF3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo assay, GI50 = 0.19 μM. | 28282122 | ||
| BAF3 | Function assay | 72 hrs | Inhibition of Tel-fused INSR (unknown origin) expressed in mouse BAF3 cells assessed as growth inhibition after 72 hrs by CellTiter-Glo assay, GI50 = 0.29 μM. | 28282122 | ||
| BAF3 | Growth inhibition assay | 72 hrs | Growth inhibition of mouse BAF3 cells after 72 hrs by CellTiter-Glo assay, GI50 = 1.9 μM. | 28282122 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 6 mg/mL
(10.77 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 557.04 | Formula | C30H29ClN6O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 698387-09-6 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | HKI-272 | Smiles | CCOC1=C(C=C2C(=C1)N=CC(=C2NC3=CC(=C(C=C3)OCC4=CC=CC=N4)Cl)C#N)NC(=O)C=CCN(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
HER2
(Cell-free assay) 59 nM
EGFR
(Cell-free assay) 92 nM
KDR
(Cell-free assay) 800 nM
Src
(Cell-free assay) 1.4 μM
|
|---|---|
| In vitro |
Neratinib weakly inhibits tyrosine kinases KDR and Src with IC50 of 0.8 μM and 1.4 μM, respectively, being 14- and 24-fold less active compared with HER2. This compound displays no activity against other serine-threonine kinases such as Akt, cyclin D1/cdk4, cyclin E/cdk2, cyclin B1/cdk1, IKK-2, MK-2, PDK1, c-Raf, and Tpl-2, as well as the tyrosine kinase c-Met. It selectively inhibits the proliferation of 3T3 cells transfected with the HER2 (3T3/neu), as well as two other HER-2-overexpressing SK-Br-3 and BT474 cells with IC50 values of 2-3 nM, displaying >230-fold potency compared with non-transfected 3T3 cells as well as MDA-MB-435 and SW620 which are EGFR- and HER2-negative. This chemical also inhibits the proliferation of EGFR-dependent A431 cells with an IC50 of 81 nM. It reduces HER2 receptor autophosphorylation in BT474 cells with an IC50 of 5 nM, and EGF-dependent phosphorylation of EGFR in A431 cells with IC50 of 3 nM. Blocking of HER-2 by this compound results in inhibition of downstream MAPK and Akt pathways with IC50 of 2 nM, more potently than Trastuzumab. It inhibits the cyclin D1 expression and the phosphorylation of the Rb-susceptibility gene production in BT474 cells with IC50 of 9 nM, leading to G1-S arrest and ultimately decreased cell proliferation.
|
| Kinase Assay |
Cell-free autophosphorylation assay using time-resolved fluorometry
|
|
Neratinib is prepared as 10 mg/mL stocks in DMSO and diluted in 25 mM HEPES (pH 7.5; 0.002 ng/mL-20 μg/mL). Purified recombinant COOH-terminal fragments of HER2 (amino acids 676-1255) or epidermal growth factor receptor (EGFR) (amino acids 645-1186) [diluted in 100 mM HEPES (pH 7.5) and 50% glycerol] is incubated with increasing concentrations of this compound in 4 mM HEPES (pH 7.5), 0.4 mM MnCl2, 20 μM sodium vanadate, and 0.2 mM DTT for 15 minutes at room temperature in 96-well ELISA plates. The kinase reaction is initiated by the addition of 40 μM ATP and 20 mM MgCl2 and allowed to proceed for 1 hour at room temperature. Plates are washed, and phosphorylation is detected using Europium-labeled anti-phospho-tyrosine antibodies (15 ng/well). After washing and enhancement steps, signal is detected using a Victor2 fluorescence reader (excitation wavelength 340 nm, emission wavelength 615 nm). The concentration of this chemical that inhibits receptor phosphorylation by 50% (IC50) is calculated from inhibition curves.
|
|
| In vivo |
Oral administration of Neratinib significantly inhibits the growth of 3T3/neu xenografts, with inhibition of 34%, 53%, 98%, and 98% at dose of 10, 20, 40, and 80 mg/kg/day, respectively. Consistent with the inhibition of HER-2 phosphorylation by 84% within 1 hour of administration at 40 mg/kg/day, this compound inhibits the growth of BT474 xenografts by 70-82%, 67%, and 93% at dose of 5, 10, and 40 mg/kg/day, respectively. It is also effective against SK-OV-3 xenografts with inhibition of 31% and 85% at 5 and 60 mg/kg/day, respectively. This chemical is less potent against EGFR-dependent A431 xenografts than HER-2-dependent tumors, with 32% and 44% inhibition at 5 and 20 mg/kg/day, respectively. It displays little activity against MCF-7 and MX-1 xenografts expressing low levels of HER-2 and EGFR, with only 28% inhibition at 80 mg/kg/day, suggesting that it has selective activity for cells expressing HER-2 or EGFR.
|
References |
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | p-ERBB2 / ERBB2 / p-ERBB3 / ERBB3 / p-EGFR / p-90RSK pHER2 / HER2 / pAKT / AKT / pERK / ERK |
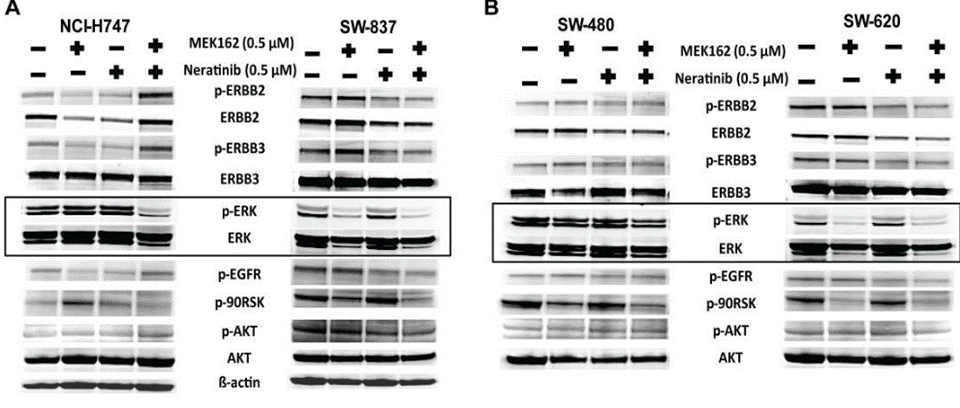
|
30118499 |
| Growth inhibition assay | Cell viability |
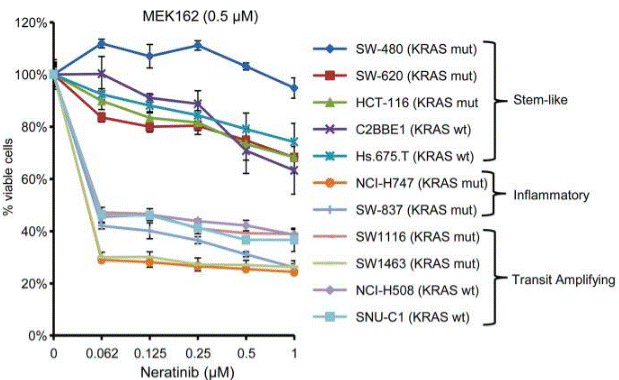
|
30118499 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05388149 | Recruiting | Breast Cancer|HER2-positive Breast Cancer |
University Health Network Toronto |
December 6 2022 | Phase 2 |
| NCT05252988 | Recruiting | Early-stage Breast Cancer|HER2 Positive Breast Cancer|Hormone Receptor Positive |
Spanish Breast Cancer Research Group|Puma Biotechnology Inc.|Pierre Fabre Laboratories |
August 31 2022 | Phase 2 |
| NCT04856475 | Withdrawn | Breast Cancer|Brain Metastases |
Jules Bordet Institute |
November 24 2021 | Phase 2 |
| NCT04781374 | Withdrawn | Metastatic Prostate Adenocarcinoma|Castration-resistant Prostate Cancer|Prostate Cancer|Prostate Cancer Metastatic |
Beth Israel Deaconess Medical Center|Puma Biotechnology Inc.|Dana-Farber Cancer Institute |
May 21 2021 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































