research use only
Flumazenil GABA Receptor antagonist
Cat.No.S1332
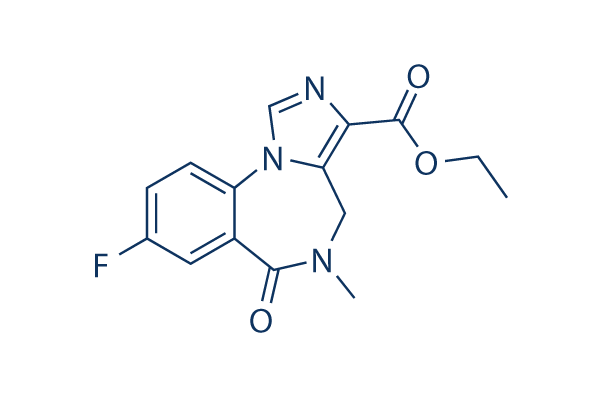
Chemical Structure
Molecular Weight: 303.29
Quality Control
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(329.71 mM)
Warmed with 50°C water bath;
Ultrasonicated;
Ethanol : 3 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 303.29 | Formula | C15H14FN3O3 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 78755-81-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | RO 15-1788 | Smiles | CCOC(=O)C1=C2CN(C(=O)C3=C(N2C=N1)C=CC(=C3)F)C | ||
Mechanism of Action
| Targets/IC50/Ki |
GABAA receptor
|
|---|---|
| In vivo |
Flumazenil interacts at the central benzodiazepine receptor to antagonize or reverse the behavioral, neurologic, and electrophysiologic effects of benzodiazepine agonists and inverse agonists. This compound is of some benefit in hepatic encephalopathy, but until well-designed clinical trials are conducted, hepatic encephalopathy must be considered an investigational indication for flumazenil. It has been shown to reverse sedation caused by intoxication with benzodiazepines alone or benzodiazepines in combination with other agents, but it should not be used when cyclic antidepressant intoxication is suspected. This chemical (1 mg/kg) induces a strong anxiolytic effect in BALB/c mice tested in the elevated plus maze and light/dark test. It (10 mg/kg) effectively prevents the reduction produced by allopregnanolone in rats. This compound (5-20 mg/kg) antagonizes the anticonvulsant and adverse effects of diazepam but not GYKI 52466 in mice. It slightly reduces the anticonvulsant activity of NBQX in the MES model but not in the PTZ test. This chemical (3.0 mg/kg) blocks the changes withdrawal from chronic ethanol treatment, which leads to a decrease in open arm time and percent open arm entries.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06275594 | Not yet recruiting | Midazolam|Remimazolam |
Yeungnam University Hospital |
March 1 2024 | Phase 2 |
| NCT05939674 | Recruiting | Flumazenil Adverse Reaction|Remimazolam|Hip Joint|Elderly Patients |
Pusan National University Yangsan Hospital |
September 19 2023 | Not Applicable |
| NCT05841667 | Recruiting | Adult|Middle Aged|Elective Surgical Procedures |
Korea University Guro Hospital |
September 6 2023 | -- |
| NCT05220462 | Recruiting | Tooth Extraction |
Guy''s and St Thomas'' NHS Foundation Trust |
March 9 2022 | Phase 3 |
| NCT05025410 | Completed | Myoma;Uterus|Polyp Endometrium|Unspecified Condition Associated With Female Genital Organs and Menstrual Cycle |
Seoul National University Bundang Hospital |
November 1 2021 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































