research use only
Clopidogrel (SR-25990C) Bisulfate P2 Receptor modulator
Cat.No.S1415
Chemical Structure
Molecular Weight: 419.9
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) |
|---|---|
| Other P2 Receptor Inhibitors | A-438079 Hydrochloride A-804598 MRS 2578 A-740003 5-BDBD JNJ-47965567 BX430 Eliapixant Aurintricarboxylic acid A-317491 |
Solubility
|
In vitro |
DMSO
: 83 mg/mL
(197.66 mM)
Ethanol : 83 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 419.9 | Formula | C16H16ClNO2S.H2SO4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 120202-66-6 | Download SDF | Storage of Stock Solutions |
|
|
Mechanism of Action
| Targets/IC50/Ki |
P2Y12
|
|---|---|
| In vitro |
Clopidogrel is converted to its active metabolite by cytochrome P450 (CYP) enzymes. Clopidogrel (1 μM) also inhibits EGF-stimulated EGF receptor, PERK expression, and cell proliferation in RGM-1 cells (P<0.05), and causes much less inhibition of EGF-stimulated cell proliferation in EGF receptor over-expressed RGM-1 cells than in RGM-1 cells (22% vs. 32% reduction). Clopidogrel increases blood vessel number, reduces polymorphonuclear count and decreases attachment and bone loss, also decreases osteoclast number in rats submitted or not to periodontal repair. Clopidogrel decreases CXCL4, CXCL12 and PDGF content compared with saline-treated rats, without affecting CXCL5.
|
| In vivo |
Clopidogrel (2mg and 10mg/kg/day) significantly decreases ulcer-induced gastric epithelial cell proliferation and ulcer-stimulated expressions of EGF receptor and phosphorylated extracellular signal-regulated kinase (PERK) at the ulcer margin of rats. Clopidogrel improves endothelial function and NO bioavailability in rats with congestive heart failure. Clopidogrel-treated Congestive heart failure (CHF) rat displays enhances phosphorylation of AKT and eNOS. The clopidogrel/aspirin combination shows only additive-type effects on bleeding time prolongation induced by ear transection in the rabbit, therefore showing that combined inhibition of cyclooxygenase and ADP's effects provide a marked enhanced antithrombotic efficacy.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | ATF2 / ATF3 / ATF4 / ATF6 TRIB3 / CHOP |
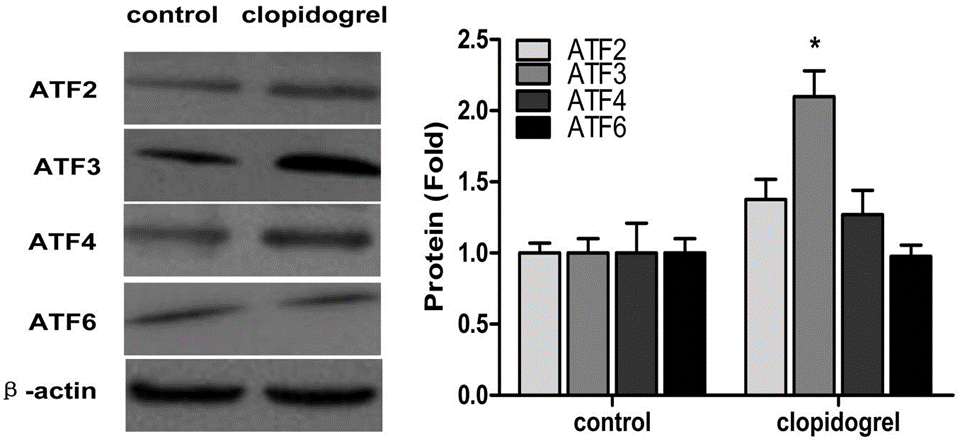
|
24058556 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05723926 | Not yet recruiting | Atrial Fibrillation|Oral Anticoagulation|Stroke|Implant |
Javelin Medical|Population Health Research Institute |
January 20 2025 | Not Applicable |
| NCT06047002 | Recruiting | Peripheral Arterial Disease |
University of Leicester |
September 29 2022 | -- |
| NCT05166538 | Unknown status | Multi Vessel Coronary Artery Disease |
Yonsei University |
February 1 2022 | Phase 4 |
| NCT05162053 | Completed | Acute Coronary Syndrome |
Jiangsu vcare pharmaceutical technology co. LTD |
December 9 2021 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































