research use only
Candesartan Cilexetil Angiotensin Receptor antagonist
Cat.No.S2037
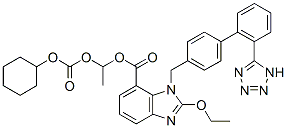
Chemical Structure
Molecular Weight: 610.66
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas |
|---|---|
| Other Angiotensin Receptor Inhibitors | PD123319 ML221 A-779 Fimasartan Olodanrigan (EMA401) Buloxibutid AVE 0991 |
Solubility
|
In vitro |
DMSO
: 122 mg/mL
(199.78 mM)
Ethanol : 4 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 610.66 | Formula | C33H34N6O6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 145040-37-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | TCV-116 | Smiles | CCOC1=NC2=CC=CC(=C2N1CC3=CC=C(C=C3)C4=CC=CC=C4C5=NNN=N5)C(=O)OC(C)OC(=O)OC6CCCCC6 | ||
Mechanism of Action
| Targets/IC50/Ki |
Ang II receptor
0.26 nM
|
|---|---|
| In vitro |
Candesartan blocks the effects of angiotensin II at the angiotensin II type 1 (AT1) receptor. Candesartan cilexetil is a prodrug that is activated to candesartan by ester hydrolysis during gastrointestinal absorption.
|
| In vivo |
Candesartan improves the functional markers in a dose-dependent manner and also upregulates Ang (1-7), ACE2 and mas1 in the myocardium of DCM rats. Candesartan reduces various ER stress and apoptosis markers and the number apoptotic cells in the Candesartan treated rats. Candesartan cilexetil shows angiotensin-II blocking action in a dose-dependent manner in rats with dilated cardiomyopathy. Candesartan cilexetil reduces the left ventricular end-diastolic pressure and heart weight/body weight ratio, the area of myocardial fibrosis and expressions of transforming growth factor-beta1 and collagen-III mRNA. Candesartan cilexetil (1 mg/kg, p.o.) and enalapril (10 mg/kg, p.o.) reduces blood pressure to the same extent 5 hours after administration on the 1st and the 7th day. Candesartan cilexetil significantly increases renal blood flow without any changes in the cardiac index. TCV-116 and enalapril also tends to increase splanchnic blood flow following the 1st dose but not the 7th dose. Candesartan cilexetil is absorbed from the small intestine and hydrolyzed completely to the pharmacologically active metabolite M-I during absorption process.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02609711 | Unknown status | Healthy Male Subjects |
Ahn-Gook Pharmaceuticals Co.Ltd |
November 2015 | Phase 1 |
| NCT00984529 | Completed | Chronic Heart Failure |
AstraZeneca |
September 2009 | -- |
| NCT00844324 | Completed | Hypertension |
AstraZeneca |
March 2009 | Phase 1 |
| NCT00244595 | Completed | Hypertension |
AstraZeneca |
September 2003 | Phase 3 |
| NCT00244634 | Completed | Pediatric Hypertension |
AstraZeneca |
September 2003 | Phase 3 |
| NCT00242346 | Completed | Proteinuria |
AstraZeneca |
April 2003 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































