research use only
Fimasartan Angiotensin Receptor antagonist
Cat.No.S4975
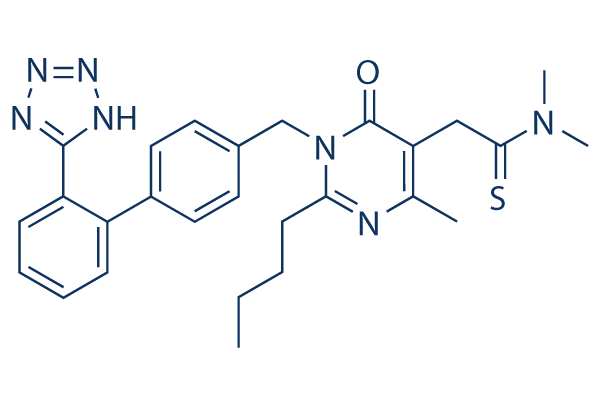
Chemical Structure
Molecular Weight: 501.65
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas |
|---|---|
| Other Angiotensin Receptor Inhibitors | PD123319 ML221 A-779 Olodanrigan (EMA401) Buloxibutid AVE 0991 |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(199.34 mM)
Ethanol : 24 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 501.65 | Formula | C27H31N7OS |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 247257-48-3 | -- | Storage of Stock Solutions |
|
|
| Synonyms | Kanarb | Smiles | CCCCC1=NC(=C(C(=O)N1CC2=CC=C(C=C2)C3=CC=CC=C3C4=NNN=N4)CC(=S)N(C)C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
AT1 receptor
|
|---|---|
| In vitro |
Fimasartan is a selective AT1 receptor antagonist. The concentration that inhibits the binding of [125I]Ang II to the AT1 receptor from rat adrenal cortex by 50% (IC50) is 0.13 nM. This compound potently attenuates myocardial apoptotic death in reperfused rat heart and H9c2 cells. It could attenuate mitochondrial damage which is associated with minimizing the reduction in Bcl-2 and connected with the reduction in p53 and activation of Akt and GSK-3β, and inhibiting mitochondrial Ca2+ overload by suppressing the ICa,L and MCU. |
| In vivo |
In various animal models including renal hypertensive rats, spontaneously hypertensive rats and Beagle dogs, fimasartan effectively reduces blood pressure in a dose-dependent manner following single or repeated oral and intravenous administration. This compound does not affect general behavior, respiratory rate or tidal volume in experimental animals, and shows no adverse findings in the human ether-a-go-go-related gene (hERG) test or monkey telemetry study. A number of general toxicity, carcinogenic toxicity, genetic toxicity, and developmental toxicity studies given either orally or intravenously in various species including mice, rats, monkeys and dogs are conducted and these preclinical results demonstrate the safety and tolerability of this chemical for long-term clinical use and offer sufficient safety margins to support the expected human therapeutic dose. It is rapidly absorbed following oral administration with the time to peak plasma concentration (Tmax) ranging 0.5-3 h and the terminal half-life (t1/2) being 5-16 h at doses of 20 to 480 mg in healthy subjects. Similar results are obtained in patients with hypertension, i.e., Tmax ranges 0.5-1.3 h and t1/2 is 7-10 h following this compound administration at doses 20-180 mg in the subsequent phase II study. The urinary excretion of this chemical is low, with the overall urinary excretion of unchanged drug over the first 24 h after dosing being less than 3% of the administered dose. It undergoes nonrenal elimination with minimal metabolism. This compound treatment before myocardial ischemia significantly attenuates reperfusion injury and apoptotic changes, possibly by suppressing mitochondrial damage during reperfusion. In various preclinical studies, including aortic balloon injury, myocardial infarct ischemia/reperfusion, doxorubicin cardiotoxicity, and ischemic stroke models, this chemical shows anti-inflammatory and organ-protecting effects. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02994745 | Completed | Hypertension Hyperlipidemia |
Boryung Pharmaceutical Co. Ltd |
December 23 2016 | Phase 1 |
| NCT02920047 | Completed | Hypertension |
Boryung Pharmaceutical Co. Ltd |
October 2016 | Phase 1 |
| NCT02995720 | Completed | Hypertension Hyperlipidemia |
Boryung Pharmaceutical Co. Ltd |
August 26 2016 | Phase 1 |
| NCT03231293 | Completed | Hypertension|Ischemic Stroke|Transient Ischemic Attack |
Boryung Pharmaceutical Co. Ltd |
July 28 2016 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































