research use only
Zanamivir Neuraminidase inhibitor
Cat.No.S3007
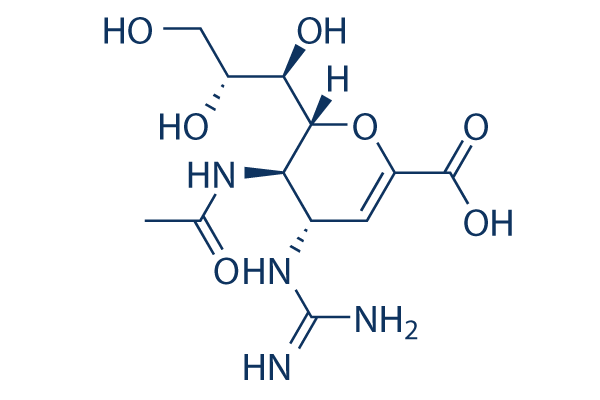
Chemical Structure
Molecular Weight: 332.31
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Other Neuraminidase Inhibitors | Laninamivir Octanoate Laninamivir |
Solubility
|
In vitro |
Water : 25 mg/mL
DMSO
: 2 mg/mL
(6.01 mM)
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 332.31 | Formula | C12H20N4O7 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 139110-80-8 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | GG167,GR 121167X | Smiles | CC(=O)NC1C(C=C(OC1C(C(CO)O)O)C(=O)O)N=C(N)N | ||
Mechanism of Action
| Targets/IC50/Ki |
neuraminidase
|
|---|---|
| In vitro |
Zanamivir, a novel neuraminidase inhibitor, is effective at decreasing replication of the virus in vitro. This compound results in reduced sensitivity in influenza A/H1N1 variant His274Asn, as well as His274Gly, His274Ser, and His274Gln. It blocks influenza neuraminidase and prevents the cleavage of sialic acid residues, thus interfering with progeny virus dispersement within the mucosal secretions and reducing viral infectivity. This agent prevents hemadsorption and fusion of persistently infected cells with uninfected cells. It reduces the number (but not the area) of plaques if present only during the adsorption period and reduces plaque area (but not number) if added only after the 90-min adsorption period in plaque assays. The compound also reduces the area of plaques formed by a neuraminidase-deficient variant, confirming that its interference with cell-cell fusion is unrelated to inhibition of neuraminidase activity. It has no effect on hemadsorption but does inhibit HA2b-red blood cell fusion, as judged by both lipid mixing and content mixing. |
| In vivo |
Zanamivir reduces lung titers of the virus and decreases morbidity and mortality in a model of lethal challenge in mice. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04597437 | Recruiting | Dengue Fever |
George Washington University|Naval Medical Research Center|Clinica de la Costa|Global Disease Research |
March 15 2024 | Early Phase 1 |
| NCT04494412 | Recruiting | Influenza Human|Arthralgia |
GlaxoSmithKline |
November 21 2022 | Phase 2 |
| NCT02377401 | Completed | Influenza Human |
GlaxoSmithKline |
April 28 2015 | Phase 1 |
| NCT01527110 | Completed | Influenza Human |
GlaxoSmithKline |
January 1 2012 | Phase 3 |
| NCT01353768 | Completed | Infections Respiratory Tract |
GlaxoSmithKline |
July 2011 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































