|
How to Cite 1. For In-Text Citation (Materials & Methods): 2. For Key Resources Table: |
||
|
Toll Free: (877) 796-6397 -- USA and Canada only -- |
Fax: +1-832-582-8590 Orders: +1-832-582-8158 |
Tech Support: +1-832-582-8158 Ext:3 Please provide your Order Number in the email. We strive to reply to |
Technical Data
| Formula | C12H20N4O7 |
||||||
| Molecular Weight | 332.31 | CAS No. | 139110-80-8 | ||||
| Solubility (25°C)* | In vitro | DMSO | 66 mg/mL (198.6 mM) | ||||
| Water | 36 mg/mL (108.33 mM) | ||||||
| Ethanol | Insoluble | ||||||
| In vivo (Add solvents to the product individually and in order) |
|
||||||
|
* <1 mg/ml means slightly soluble or insoluble. * Please note that Selleck tests the solubility of all compounds in-house, and the actual solubility may differ slightly from published values. This is normal and is due to slight batch-to-batch variations. * Room temperature shipping (Stability testing shows this product can be shipped without any cooling measures.) |
|||||||
Preparing Stock Solutions
Biological Activity
| Description | Zanamivir (GG167,GR 121167X) is a neuraminidase inhibitor used in the treatment and prophylaxis of influenza caused by influenza A virus and influenza B virus. | |
|---|---|---|
| Targets |
|
|
| In vitro | Zanamivir, a novel neuraminidase inhibitor, is effective at decreasing replication of the virus in vitro. This compound results in reduced sensitivity in influenza A/H1N1 variant His274Asn, as well as His274Gly, His274Ser, and His274Gln. It blocks influenza neuraminidase and prevents the cleavage of sialic acid residues, thus interfering with progeny virus dispersement within the mucosal secretions and reducing viral infectivity. This agent prevents hemadsorption and fusion of persistently infected cells with uninfected cells. It reduces the number (but not the area) of plaques if present only during the adsorption period and reduces plaque area (but not number) if added only after the 90-min adsorption period in plaque assays. The compound also reduces the area of plaques formed by a neuraminidase-deficient variant, confirming that its interference with cell-cell fusion is unrelated to inhibition of neuraminidase activity. It has no effect on hemadsorption but does inhibit HA2b-red blood cell fusion, as judged by both lipid mixing and content mixing. |
|
| In vivo | Zanamivir reduces lung titers of the virus and decreases morbidity and mortality in a model of lethal challenge in mice. |
Protocol (from reference)
References
|
Customer Product Validation
-
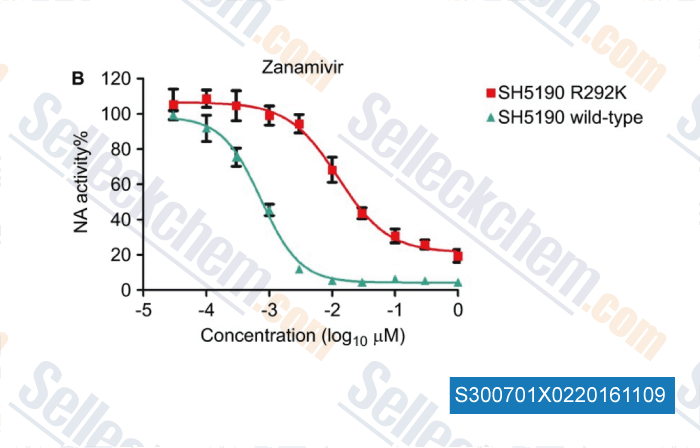
, , Emerg Microbes Infec, 2014, 3:e78.
Selleck's Zanamivir Has Been Cited by 3 Publications
| Siglec-G Deficiency Ameliorates Hyper-Inflammation and Immune Collapse in Sepsis via Regulating Src Activation [ Front Immunol, 2019, 10:2575] | PubMed: 31781099 |
| Drug susceptibility profile and pathogenicity of H7N9 influenza virus (Anhui1 lineage) with R292K substitution. [ Emerg Microbes Infect, 2014, 3(11):e78] | PubMed: 26038501 |
| Drug susceptibility profile and pathogenicity of H7N9 influenza virus (Anhui1 lineage) with R292K substitution. [Zhang XN, et al. Emerg Microbes Infec, 2014, 3, e78] |
RETURN POLICY
Selleck Chemical’s Unconditional Return Policy ensures a smooth online shopping experience for our customers. If you are in any way unsatisfied with your purchase, you may return any item(s) within 7 days of receiving it. In the event of product quality issues, either protocol related or product related problems, you may return any item(s) within 365 days from the original purchase date. Please follow the instructions below when returning products.
SHIPPING AND STORAGE
Selleck products are transported at room temperature. If you receive the product at room temperature, please rest assured, the Selleck Quality Inspection Department has conducted experiments to verify that the normal temperature placement of one month will not affect the biological activity of powder products. After collecting, please store the product according to the requirements described in the datasheet. Most Selleck products are stable under the recommended conditions.
NOT FOR HUMAN, VETERINARY DIAGNOSTIC OR THERAPEUTIC USE.
