research use only
Valganciclovir HCl CMV inhibitor
Cat.No.S4050
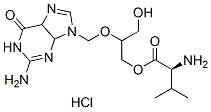
Chemical Structure
Molecular Weight: 390.82
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite Reverse Transcriptase HIV |
|---|---|
| Other CMV Inhibitors | Valaciclovir HCl Bisindolylmaleimide IV Brivudine (BVDU) |
Solubility
|
In vitro |
DMSO
: 78 mg/mL
(199.58 mM)
Water : 78 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 390.82 | Formula | C14H22N6O5.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 175865-59-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC(C)C(C(=O)OCC(CO)OCN1C=NC2=C1N=C(NC2=O)N)N.Cl | ||
Mechanism of Action
| In vitro |
Valganciclovir hydrochloride is a hydrochloride salt of the L-valyl ester of ganciclovir that exists as a mixture of two diastereomers. After administration, these diastereomers are rapidly converted to ganciclovir by hepatic and intestinal esterases. In cytomegalovirus (CMV)-infected cells, ganciclovir is initially phosphorylated to the monophosphate form by viral protein kinase, then it is further phosphorylated via cellular kinases to produce the triphosphate form. This triphosphate form is slowly metabolized intracellularly. The phosphorylation is dependent upon the viral kinase and occurs preferentially in virus-infected cells. The virustatic activity of ganciclovir is due to the inhibition of viral DNA synthesis by ganciclovir triphosphate. Ganciclovir triphosphate is incorporated into the DNA strand replacing many of the adenosine bases. This results in the prevention of DNA synthesis, as phosphodiester bridges can longer to be built, destabilizing the strand. Ganciclovir inhibits viral DNA polymerases more effectively than it does cellular polymerase, and chain elongation resumes when ganciclovir is removed.
|
|---|---|
| In vivo |
After oral administration, intestinal and hepatic esterases hydrolyze both diastereomers to ganciclovir, which inhibits replication of the human cytomegalovirus. Valganciclovir is well absorbed from the gastrointestinal tract and the absolute bioavailability from valganciclovir tablets (following administration with food) is approximately 60%.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06407232 | Not yet recruiting | Cytomegalovirus Infections|Kidney Transplant Infection|Pancreas Transplant |
University of Wisconsin Madison|Merck Sharp & Dohme LLC |
May 2024 | Phase 3 |
| NCT06034925 | Recruiting | Transplant Complication|CMV |
Medical University of South Carolina|Takeda |
November 6 2023 | Phase 4 |
| NCT03301415 | Terminated | Congenital Cytomegalovirus Infection |
National Institute of Allergy and Infectious Diseases (NIAID) |
August 21 2019 | Phase 2 |
| NCT03698435 | Unknown status | Cytomegalovirus Infections |
University Medical Center Groningen |
May 25 2018 | -- |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































