research use only
Ticagrelor P2 Receptor antagonist
Cat.No.S4079
Chemical Structure
Molecular Weight: 522.57
Quality Control
| Related Targets | Adrenergic Receptor AChR 5-HT Receptor COX Calcium Channel Histamine Receptor Dopamine Receptor GABA Receptor TRP Channel Cholinesterase (ChE) |
|---|---|
| Other P2 Receptor Inhibitors | A-438079 Hydrochloride A-804598 MRS 2578 A-740003 5-BDBD JNJ-47965567 BX430 Eliapixant Aurintricarboxylic acid A-317491 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| H9c2 | Function assay | 0.1, 0,3 and 1 μM | 24 h | effective in reducing NCX1 reverse activity when lower concentrations | ||
| EAhy926 | Apoptosis assay | 40 μM and 60 μM | decrease ox-LDL-induced apoptosis, particularly at a higher concentration | |||
| AsPC-1 | Function assay | 10 μM | 2 h | ticagrelor negated the platelet releasate effect on Akt, Erk activation and Slug upregulation | ||
| BxPC-3 | Function assay | 10 μM | 2 h | ticagrelor negated the platelet releasate effect on Akt, Erk activation and Slug upregulation | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(191.36 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 522.57 | Formula | C23H28F2N6O4S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 274693-27-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | AZD6140, AR-C 126532XX | Smiles | CCCSC1=NC(=C2C(=N1)N(N=N2)C3CC(C(C3O)O)OCCO)NC4CC4C5=CC(=C(C=C5)F)F | ||
Mechanism of Action
| Features |
First-in-class of a new type of P2Y12 antagonist known as cyclopentyl-triazolo-pyrimidines.
|
|---|---|
| Targets/IC50/Ki |
P2Y12
2 nM(Ki)
|
| In vitro |
Ticicagrelor is an active drug which, does not require metabolic activation after intestinal absorption. It does not compete directly with ADP at the ADP binding site but occupies an adjacent binding site and acts in an allosteric way, resulting in a reversible conformational change of the receptor. This compound binds reversibly to the receptor and exhibits rapid onset and offset of effect. Binding studies in rh-P2Y12 receptor-transfected CHO-K1 cells indicate that this compound exhibits potent, rapid, and reversible binding, with a Kd of 10.5 nM, a kon (association constant) of 0.00011/(nM•s), a koff (dissociation constant) of 0.00087/s, and half-life values of 4 min for binding and 14 min for unbinding, indicating that the magnitude of platelet inhibition is dependent on concentrations of drug available to bind platelets. This chemical moderately inhibits CYP2C9 activity in human liver microsomes, while exhibiting little or no inhibition of CYP1A2, CYP2B6, CYP2C8, CYP2C19, CYP2D6, and CYP2E1. In human liver microsomes, it inhibits midazolam 4-hydroxylation, while activating 1_-hydroxylation of midazolam. Evaluated in fresh human hepatocytes, this compound is not an inducer of CYP1A2 or CYP3A4. |
| Kinase Assay |
Binding assays using P2Y12-transfected CHO-K1 or humanplatelet membranes
|
|
Membranes (5 μg of protein) are added to a 96-well plate containing [125I]AZ11931285 (125 pM), [3H]ADP (10 nM), or [33P]2MeS-ADP (62.5 pM), the required concentration of competitor, and a sufficient volume of buffer (50 mM Tris, 5 mM MgCl2, 50 mM NaCl, and 0.1% nucleotide-free BSA, pH 7.4) to bring the total volume in each well to 200 μL. Binding studies using platelet membranes and [3H]ADP are performed in the presence of 100 μM (final concentration) MRS2179 to prohibit binding to P2Y1. The signal-to-noise ratios for P2Y12-transfected CHO-K1 cells are approximately 14 for [3H]ADP (specific signal: 895 c.p.m.), 24 for [33P]2MeSADP (specific signal: 3308 c.p.m.), and 24 for [125I]AZ11931285 (specific signal: 3308 c.p.m.). For the studies using platelet membranes, the signal-to-noise ratios are approximately 2 for [33P]2MeS-ADP and [3H]ADP and 1.5 for [125I]AZ11931285, with a specific signal between 100 and 400 c.p.m. In this study, an incubation time of 1 h at 30 癈 is used to allow full equilibrium to be achieved. Thereafter, free radioligand is separated from bound radioligand and counted as described above.
|
|
| In vivo |
Absorption of ticagrelor is rapid with t max of 1.3-2 h. And the Cmax and area under the plasma concentration-time curve from time 0 to infinity increases in an apparently dose-proportional manner over the dose range studied, indicating linear pharmacokinetics. The mean terminal-phase half-life (t1/2) is approximately 7-8.5 h for this compound. Inhibition of platelet aggregation (IPA) is dose related and is nearly complete at 2 h at doses of 100-400 mg. This chemical is well tolerated, with no serious or doserelated adverse events or notable changes in laboratory values observed. |
References |
|
Applications
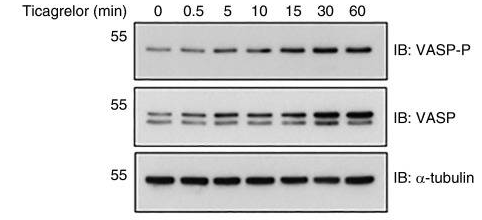
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | VASP-P / VASP |
|
27694321 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05774431 | Recruiting | Acute Myocardial Infarction |
University Hospital Heidelberg|AstraZeneca |
March 13 2023 | -- |
| NCT05283356 | Recruiting | Severe Aortic Valve Stenosis|Aortic Valve Stenosis|Transcatheter Aortic Valve Replacement (TAVR)|Transcatheter Aortic Valve Implantation (TAVI) |
Fundacin Biomedica Galicia Sur |
January 21 2022 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































