research use only
Raltitrexed Thymidylate Synthase inhibitor
Cat.No.S1192
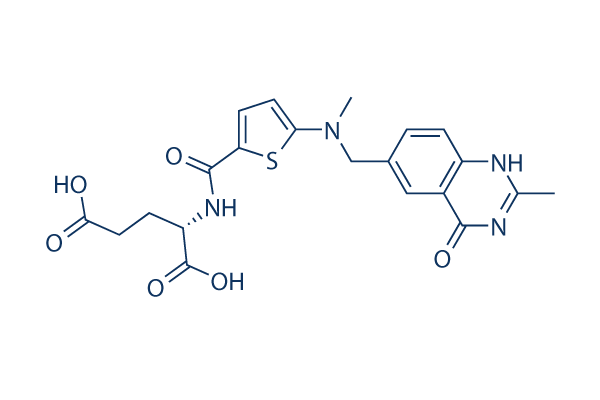
Chemical Structure
Molecular Weight: 458.49
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase |
|---|---|
| Featured Inhibitors | Y-27632 Dihydrochloride SB431542 CHIR-99021 (Laduviglusib) RMC-7977 RMC-6236 (Daraxonrasib) MRTX1133 MG132 Z-VAD-FMK VT3989 IAG933 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| human KB cells | Cytotoxicity assay | 96 h | Cytotoxicity against human KB cells assessed as cell growth inhibition incubated up to 96 hrs by Celltiter-blue cell viability assay, IC50=5.9 nM | |||
| PC43-10 cells | Function assay | 96 h | Inhibition of RFC (unknown origin) expressed in Chinese hamster PC43-10 cells assessed as cell growth inhibition incubated up to 96 hrs by Celltiter-blue cell viability assay, IC50=6.3 nM | |||
| L1210 cell line | Growth inhibition assay | Growth inhibition of murine tumor L1210 cell line, IC50=9 nM | ||||
| human IGROV1 cells | Proliferation assay | Antiproliferative activity against human RFC and FRalpha expressing human IGROV1 cells, IC50=12.6 nM | ||||
| RT16 cells | Proliferation assay | 96 h | Antiproliferative activity against chinese hamster RT16 cells expressing human FRalpha assessed as reduction of viable cells after 96 hrs, IC50=15 nM | |||
| chinese hamster D4 cells | Proliferation assay | 96 h | Antiproliferative activity against chinese hamster D4 cells expressing human FRbeta assessed as reduction of viable cells after 96 hrs, IC50=22 nM | |||
| human KB cells | Cytotoxicity assay | 200 nM | 96 h | Cytotoxicity against human KB cells assessed as cell growth inhibition incubated up to 96 hrs in presence of 200 nM folic acid by Celltiter-blue cell viability assay, IC50=22 nM | ||
| R2/PCFT4 cells | Function assay | 96 h | Inhibition of PCFT (unknown origin) expressed in Chinese hamster R2/PCFT4 cells assessed as cell growth inhibition incubated up to 96 hrs by Celltiter-blue cell viability assay, IC50=0.0995 μM | |||
| R2 cells | Cytotoxicity assay | 96 h | Cytotoxicity against chinese hamster R2 cells expressing human PCFT4 after 96 hrs by CellTitre-Blue fluorescence assay | |||
| R2 cells | Growth inhibition assay | 96 h | Growth inhibition of Chinese hamster R2 cells expressing human PCFT4 after 96 hrs by CellTiter-blue assay, IC50=0.0995 μM | |||
| L1210 mouse leukemia cells | Function assay | Compound was evaluated for inhibition of thymidylate synthase, partially purified from L1210 mouse leukemia cells that overproduce thymidylate synthase due to amplification of TS gene, Ki=0.418 μM | ||||
| human HepG2 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human HepG2 cells after 48 hrs by MTT assay, IC50=1.3 μM | |||
| SGC7901 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human SGC7901 cells after 48 hrs by MTT assay, IC50=8.7 μM | |||
| MCF7 cells | Cytotoxicity assay | 48 h | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50=12.6 μM | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 92 mg/mL
(200.65 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 458.49 | Formula | C21H22N4O6S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 112887-68-0 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | ZD-1694, ICI-D1694, D1694 | Smiles | CC1=NC2=C(C=C(C=C2)CN(C)C3=CC=C(S3)C(=O)NC(CCC(=O)O)C(=O)O)C(=O)N1 | ||
Mechanism of Action
| In vitro |
Raltitrexed induces a concentration-dependent amount of double-stranded DNA breaks. This compound increases the level of Bax protein up to a factor 5 in the Lovo and LS174T cell lines containing wt p53. It leads to an increase of intracellular phosphoribosyl pyrophosphate (PRPD) in the case of the HCT-8 cell line, which suggests that the cytotoxic effects of this compound combined with 5-FU may be due to the increased formation of 5-FU nucleotides. This agent combined with SN-38 results in synergistic cytotoxicity at broad dose-effect ranges in human colon cancer cells. It is actively taken up into cells and then undergoes rapid, extensive metabolism to a series of polyglutamates, which results in potent thymidylate synthase inhibition. This chemical is delivered to the brain very quickly, and could be detected at 5 min in all brain tissues. It combined folinic acid (5FU-FA) shows a clear schedule-dependent synergistic antiproliferative interaction as demonstrated by calculating combination indexes. This compound combined with Vorinostat produces synergistic effect paralleled by evident cell cycle perturbations with major S-phase arrest. It is a specific, folate-based inhibitor of thymidylate synthase with activity in advanced colorectal cancer comparable with that of fluorouracil (5-fluorouracil) plus folinic acid. Its activity is enhanced by rapid cellular entry and polyglutamation, with the polyglutamated derivatives having approximately 100-fold greater inhibitory potency than the parent compound.
|
|---|---|
| In vivo |
Raltitrexed could be directly transported into the brain via the olfactory pathway in rats.
|
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Growth inhibition assay | Cell viability |
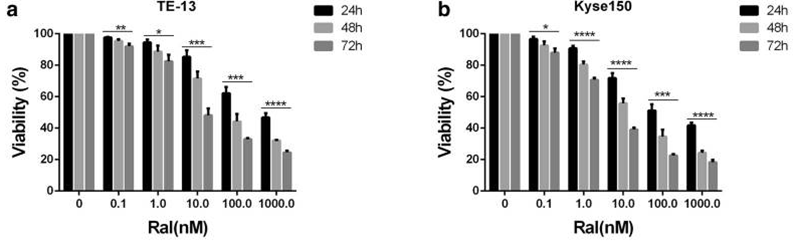
|
30820189 |
| Western blot | Cleaved caspase-3 / Bax |

|
30820189 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02618356 | Unknown status | Metastatic Colon Cancer |
Fudan University |
December 25 2015 | Phase 2 |
| NCT02821559 | Completed | Metastatic Colorectal Cancer |
Centre Hospitalier Universitaire de Besancon|Hospira now a wholly owned subsidiary of Pfizer |
July 2012 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































