research use only
Letrozole Aromatase inhibitor
Cat.No.S1235
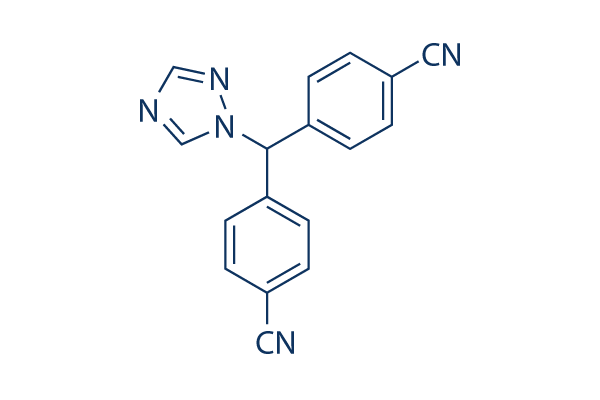
Chemical Structure
Molecular Weight: 285.3
Quality Control
| Related Targets | Adrenergic Receptor Estrogen/progestogen Receptor GPR Androgen Receptor Glucocorticoid Receptor ACE RAAS Progesterone Receptor Opioid Receptor PGES |
|---|---|
| Other Aromatase Inhibitors | Obacunone (AI3-37934) Fadrozole (CGS16949A) alpha-Naphthoflavone |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| JEG3 | Function assay | Inhibition of aromatase activity in human JEG3 cells, IC50=0.00089μM | 18590272 | |||
| JEG3 | Function assay | Inhibition of aromatase in human JEG3 cells by scintillation spectrometry, IC50=0.00089μM | 20148564 | |||
| MDA-MB-435 | Growth inhibition assay | 48 hrs | Growth inhibition of human MDA-MB-435 cells after 48 hrs by sulforhodamine B assay, GI50=0.067μM | 20950898 | ||
| IGROV1 | Growth inhibition assay | 48 hrs | Growth inhibition of human IGROV1 cells after 48 hrs by sulforhodamine B assay, GI50=0.095μM | 20950898 | ||
| MDA-MB-231 | Growth inhibition assay | 48 hrs | Growth inhibition of human MDA-MB-231cells after 48 hrs by sulforhodamine B assay, GI50=0.15μM | 20950898 | ||
| T47D | Growth inhibition assay | 48 hrs | Growth inhibition of human T47D cells after 48 hrs by sulforhodamine B assay, GI50=0.44μM | 20950898 | ||
| MCF7 | Growth inhibition assay | 48 hrs | Growth inhibition of human MCF7 cells after 48 hrs by sulforhodamine B assay, GI50=0.7μM | 20950898 | ||
| OVCAR4 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR4 cells after 48 hrs by sulforhodamine B assay, GI50=0.88μM | 20950898 | ||
| BT549 | Growth inhibition assay | 48 hrs | Growth inhibition of human BT549 cells after 48 hrs by sulforhodamine B assay, GI50=0.89μM | 20950898 | ||
| SKOV3 | Growth inhibition assay | 48 hrs | Growth inhibition of human SKOV3 cells after 48 hrs by sulforhodamine B assay, GI50=1.09μM | 20950898 | ||
| NCI/ADR-RES | Growth inhibition assay | 48 hrs | Growth inhibition of human NCI/ADR-RES cells after 48 hrs by sulforhodamine B assay, GI50=1.16μM | 20950898 | ||
| MDA-N | Growth inhibition assay | 48 hrs | Growth inhibition of human MDA-N cells after 48 hrs by sulforhodamine B assay, GI50=1.61μM | 20950898 | ||
| OVCAR5 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR5 cells after 48 hrs by sulforhodamine B assay, GI50=1.62μM | 20950898 | ||
| Hs 578T | Growth inhibition assay | 48 hrs | Growth inhibition of human Hs 578T cells after 48 hrs by sulforhodamine B assay, GI50=2.17μM | 20950898 | ||
| OVCAR8 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR8 cells after 48 hrs by sulforhodamine B assay, GI50=4.5μM | 20950898 | ||
| OVCAR3 | Growth inhibition assay | 48 hrs | Growth inhibition of human OVCAR3 cells after 48 hrs by sulforhodamine B assay, GI50=5.87μM | 20950898 | ||
| MCF7a | Cytotoxicity assay | 10 days | Cytotoxicity against human MCF7a cells expressing Tet-off-3betaHSD1-Arom assessed as inhibition of TST-stimulated cell proliferation measured after 10 days, EC50=0.000004μM | 22951074 | ||
| V79MZh | Function assay | Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxy-corticosterone as substrate, IC50=1.42μM | 23281812 | |||
| V79MZh | Function assay | Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxy-corticosterone as substrate, IC50=2.62μM | 23281812 | |||
| H295R | Function assay | 5 uM | 24 hrs | Inhibition of CYP19 in human H295R cells using [1beta-3H(N)]-androst-4-ene-3,17-dione at 5 uM after 24 hrs by tritiated water release assay | 24025069 | |
| MCF7 | Cytotoxicity assay | 72 hrs | Cytotoxicity against estrogen-dependent human MCF7 cells after 72 hrs by MTT assay, IC50=0.007μM | 24345481 | ||
| MCF7 | Cytotoxicity assay | 24 to 96 hrs | Cytotoxicity against human MCF7 cells after 24 to 96 hrs, IC50=0.02μM | 24345481 | ||
| JEG-3 | Function assay | Inhibition of aromatase (unknown origin) expressed in JEG-3 cells, IC50=0.00089μM | 25992880 | |||
| insect cells | Function assay | 30 mins | Inhibition of recombinant human CYP19 expressed in baculovirus infected insect cells using MFC as substrate measured after 30 mins by fluorometric analysis, IC50=0.0053μM | 27647367 | ||
| T47D | Function assay | 24 hrs | Inhibition of aromatase activity in human T47D cells after 24 hrs, IC50=29.5μM | 27770735 | ||
| T47D | Function assay | 24 hrs | Inhibition of aromatase activity in human T47D cells after 24 hrs, IC50=29.5μM | 28427017 | ||
| MCF7 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human MCF7 cells after 48 hrs by MTT assay, IC50=4.73μM | 29202405 | ||
| MCF7 | Growth inhibition assay | 72 hrs | Growth inhibition of human MCF7 cells incubated for 72 hrs by cell counting based method, GI50=4.1μM | 30822713 | ||
| MDA-MB-231 | Growth inhibition assay | 72 hrs | Growth inhibition of human MDA-MB-231 cells incubated for 72 hrs by cell counting based method, GI50=10μM | 30822713 | ||
| insect cells | Function assay | Inhibition of recombinant human aromatase expressed in baculovirus infected insect cells using O-benzyl fluorescein benzyl ester as substrate in presence of NADPH generating system by fluorescence based analysis, IC50=0.0019μM | 31416738 | |||
| MCF-7aro | Function assay | 1 hr | Inhibition of aromatase in human MCF-7aro cells using [1beta-3H] androstenedione as substrate incubated for 1 hr by liquid scintillation counting method, IC50=0.0019μM | 31732252 | ||
| insect cells | Function assay | 30 mins | Inhibition of human recombinant aromatase expressed in baculovirus infected insect cells using O-benzylfluorescein benzyl ester as substrate after 30 mins, IC50=0.0011μM | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 57 mg/mL
(199.78 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 285.3 | Formula | C17H11N5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 112809-51-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CGS 20267 | Smiles | C1=CC(=CC=C1C#N)C(C2=CC=C(C=C2)C#N)N3C=NC=N3 | ||
Mechanism of Action
| Targets/IC50/Ki |
Aromatase
(Cell-free assay) 0.07 nM-20 nM
|
|---|---|
| In vitro |
Letrozole potently inhibits aromatase derived from a variety of different sources including human placental microsomes, particulate fractions of human breast cancer, rat ovarian microsomes, MCF-7 cells transfected with aromatase (MCF-7Ca), JEG-3 human choriocarcinoma cells , CHO cells, hamster ovarian tissue, and particulate fractions of human breast cancer with IC50 of 11, 2, 7, 0.07, 0.07, 1.4, 20 and 0.8 nM. In the non-cellular systems, the IC50 of this compound is calculated to be 1-13 nM. It maximally inhibits estradiol production in vitro in LH-stimulated hamster ovarian tissue at 0.1 μM with an IC50 of 0.02 μM and does not significantly affect progesterone production up to 350 μM. In ACTH-stimulated rat adrenal tissue in vitro, aldosterone production is inhibited by with an IC50 of 210 μM. This chemical inhibits growth of the MCF-7 epithelial breast cancer cells in a dose-dependent way with IC50 of 1 nM. Inhibition can be observesed even at the very low concentrations tested (0.1 nM). Treatment of normal MCF-12A epithelial cells with this compound did not affect their growth even when high letrozole concentrations (100 nM) or prolonged culture times. Concurrent administration of 17-β-estradiol with it (10 nM) decreased the stimulatory effect of the enzymatic activity of MMP-2 and - 9 released by estradiol. |
| Kinase Assay |
Human placental aromatase activity
|
|
The assay is performed in a total volume of 1 mL at 37 ℃. Unless otherwise noted, the incubation mixture contains 11 nM [4- 14C] androstene-3, 17-dione ([4- 14C]A), 24 mM NADPH (tetrasodium salt Type III), the appropriate concentrations of the desired inhibitor, and 120 μg of microsomal protein. The (4- 14C)A is added as a solution in 1.7% ethanol in 0.05 M potassium phosphate buffer (pH 7.4), so that the final concentration of ethanol does not exceed 0.02% (v/v). The reaction is started by the addition of enzyme and stopped after 20 min by the addition of 7 vol of ethyl acetate. The mixture is agitated on a vortex mixer and centrifuged at 600 g for 5 min. The aqueous phase is re-extracted with 7 vol of ethyl acetate, and the combined extracts are evaporated to dryness using an Evapo-Mix. Over 99% of the radio- active of [4- 14C] added is recovered using this extraction system. The residue obtained is dissolved in 150 μL acetone, and 100 μL aliquots are chromatographed for 65 min on thin-layer plates precoated with silica gel 60 using ethyl: acetate: isooctane (140:60, v/v; system A) or toluene: chloroform: methanol (70:140:20; system B). The radioactive zones of the plate are located with a Berthold LB 2760 thin-layer scanner. The radioactive estradiol (E2) and estrone (E1) neaks are identified by comparison with authentic standards. The corresponding bonding band of silica gel is transferred to vials containing 10 mL of scintillation fluid, and counted with a 6880 Liquid Scintillation system.
|
|
| In vivo |
Letrozole inhibits aromatase in vivo with ED50 of 1-3 μg/kg p.o.. This compound displays anti-endocrine effects. It inhibits androstenedione-induced uterine hypertrophy in immature rats with ED50 of 1-3 μg/kg. In the adult female rat, this compound (0.3-1 mg/kg daily p.o., 14 days) completely interrupts ovarian cyclicity and reduces uterine weight and serum estradiol (E2) concentrations to a similar extent to that seen after ovariectomy. This chemical induces dose-dependent regression of estrogen-dependent, 9,10-dimethylbenz-a-anthracene-induced mammary tumors in adult female rats. The ED50 for it is determined to be 10 - 30 µg/kg/day, with complete inhibition at a daily dose of 10 µg/day. It produces dose-dependent inhibition of tumor growth of MCF-7 cells transfected with human aromatase gene (MCF-7Ca) implanted athymic nude mice, with complete inhibition at 20 mg/kg per day p.o.. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06143631 | Not yet recruiting | Leiomyoma Uterine|Leiomyoma|Fibroid|Fibroid Uterus |
University of California San Francisco|Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) |
May 13 2024 | Phase 4 |
| NCT05872204 | Recruiting | Low Grade Serous Ovarian Carcinoma|Adult Type Granulosa Cell Tumor |
Universitaire Ziekenhuizen KU Leuven|Kom Op Tegen Kanker|Eli Lilly and Company|European Network of Gynaecological Oncological Trial Groups (ENGOT) |
November 30 2023 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































