research use only
Bimatoprost Prostaglandin Receptor agonist
Cat.No.S1407
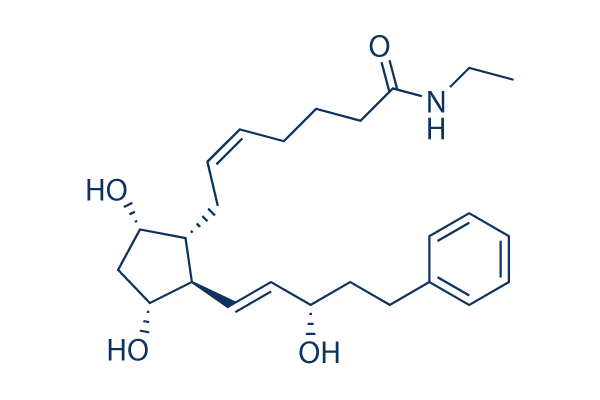
Chemical Structure
Molecular Weight: 415.57
Quality Control
| Related Targets | CXCR Hedgehog/Smoothened PKA Adrenergic Receptor AChR 5-HT Receptor Histamine Receptor Dopamine Receptor Ras KRas |
|---|---|
| Other Prostaglandin Receptor Inhibitors | PF-04418948 TG4-155 E7046 (ER-886406) Ethamsylate Grapiprant (CJ-023,423) Timapiprant Sodium Seratrodast(AA-2414) BI-671800 Setipiprant (ACT-129968) Terutroban |
Solubility
|
In vitro |
DMSO
: 83 mg/mL
(199.72 mM)
Ethanol : 83 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 415.57 | Formula | C25H37NO4 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 155206-00-1 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | AGN 192024 | Smiles | CCNC(=O)CCCC=CCC1C(CC(C1C=CC(CCC2=CC=CC=C2)O)O)O | ||
Mechanism of Action
| In vitro |
Bimatoprost mildly stimulates the rate of aqueous humor flow during the day (13%) and at night (14%), its ocular hypotensive action is due primarily to a 26% reduction in the tonographic resistance to outflow. This compound enhances the pressure-sensitive outflow pathway. It displaces [3H]prostaglandin F(2alpha) from FP receptors with K(i) of 6.31 μM. This chemical rapidly mobilizes intracellular Ca(2+) via cloned human FP receptors expressed in human embryonic kidney cells and via native FP receptors in 3T3 mouse fibroblasts with EC(50) of 2.94 μM and 2.2 μM. It up-regulates Cyr61 mRNA expression in the cat iris. This compound-induced up-regulation of Cyr61 mRNA expression is not because of the activation of the prostaglandin FP receptor but a different receptor. It consistently evokes responses in different cells within the same tissue preparation, whereas prostaglandin F(2 alpha) and 17-phenyl prostaglandin F(2 alpha) elicites signaling responses in the same cells. This chemical selectively stimulates intracellular calcium signaling in different cat iris sphincter cells. |
|---|---|
| In vivo |
Bimatoprost is the ethyl amide derivative of 17-phenyl trinor PGF2α, a potent prostaglandin FP receptor agonist. This compound elicits an immediate, robust spike in [Ca2+] that rapidly decayes back to baseline levels. It possess direct agonist activities at the rat, mouse, and human FP prostanoid receptor. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05729594 | Completed | Healthy Volunteers |
Laboratoires Thea |
February 27 2023 | Phase 1 |
| NCT04647214 | Recruiting | Open-angle Glaucoma|Ocular Hypertension |
Allergan |
March 3 2021 | -- |
| NCT04285580 | Completed | Open-Angle Glaucoma|Ocular Hypertension |
AbbVie |
June 11 2020 | Phase 3 |
| NCT03891446 | Enrolling by invitation | Open-Angle Glaucoma|Ocular Hypertension |
AbbVie |
March 27 2019 | Phase 3 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































