- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Vildagliptin DPP inhibitor
Cat.No.S3033
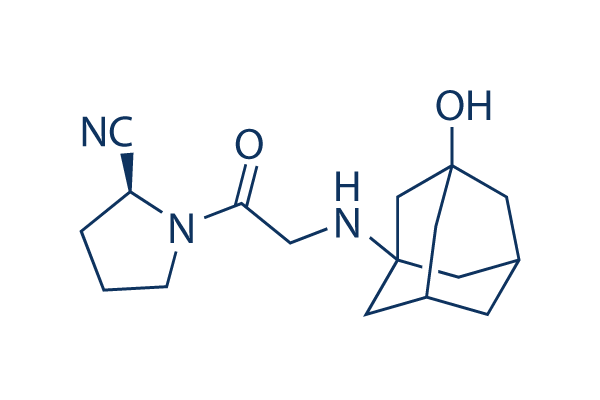
Chemical Structure
Molecular Weight: 303.4
Jump to
Quality Control
Batch:
Purity:
99.84%
99.84
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| Caco-2 cells | Function assay | Inhibition of human DPP4 expressed in Caco-2 cells, Ki=0.004 μM | ||||
| insect cells | Function assay | Inhibition of human recombinant His-tagged DPP4 expressed in insect cells, IC50=0.056 μM | ||||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 60 mg/mL
(197.75 mM)
Water : 60 mg/mL Ethanol : 60 mg/mL |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 303.4 | Formula | C17H25N3O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 274901-16-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LAF-237, NVP-LAF 237 | Smiles | C1CC(N(C1)C(=O)CNC23CC4CC(C2)CC(C4)(C3)O)C#N | ||
Mechanism of Action
| Targets/IC50/Ki |
DPP-4
(Cell-free assay) 2.3 nM
|
|---|---|
| In vitro |
Vildagliptin is the most stable DPP-IV inhibitor, binding in the S1- and S2-catalytic sites of DPP-IV, possessing a P-1 site transition-state mimetic. |
| In vivo |
Vildagliptin (orally dosed with 10 μmol/kg) is a potent, orally active inhibitor of plasma DPP-IV activity that provides increased levels of GLP-1 in an oral glucose tolerance test (OGTT) with Obese male Zucker rats. This compound orally dosed with 10 μmol/kg both significantly decreases glucose excursions and stimulates insulin secretion in Obese male Zucker rats. Maximum inhibition of plasma DPP-IV activity (95%) is observed approximately 2 hours postdose of this compound (1 μmol/kg, po) while >50% inhibition of DPP-IV is observed within 30 min postdose and persisted for >10 hours in normal Cynomolgus monkeys. This chemical (60 mg/kg) increases pancreatic beta cell mass through enhanced beta cell replication and reduced apoptosis, and the increased beta cell mass is sustained for 12 days after vildagliptin washout. This compound administrated at doses of 10 mg/kg for 32 weeks protects nerve fiber loss in streptozotocin (STZ)-induced diabetic adult male Sprague Dawley rats. |
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04081857 | Completed | Health Subjective |
Hanmi Pharmaceutical Company Limited |
June 3 2019 | Phase 1 |
| NCT04087525 | Completed | Health Subjective |
Hanmi Pharmaceutical Company Limited |
October 23 2018 | Phase 1 |
| NCT03577184 | Withdrawn | Diabetes Mellitus Type 2 |
Clinision |
July 2018 | -- |
| NCT05337969 | Completed | To Determine Bioequivalence Under Fed Conditions |
AET Laboratories Private Limited|Alfred E. Tiefenbacher (GmbH & Co. KG) |
December 2015 | Not Applicable |
| NCT05329857 | Completed | To Determine Bioequivalence Under Fed Conditions |
AET Laboratories Private Limited|Alfred E. Tiefenbacher (GmbH & Co. KG) |
December 2015 | Not Applicable |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































