research use only
Mevastatin HMG-CoA Reductase inhibitor
Cat.No.S4223
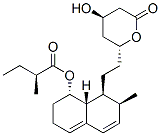
Chemical Structure
Molecular Weight: 390.51
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other HMG-CoA Reductase Inhibitors | SR-12813 Clinofibrate Cerivastatin sodium Dihydrolanosterol 7-ketocholesterol |
Solubility
|
In vitro |
DMSO
: 78 mg/mL
(199.73 mM)
Ethanol : 1 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 390.51 | Formula | C23H34O5 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 73573-88-3 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | ML-236B,Compactin | Smiles | CCC(C)C(=O)OC1CCC=C2C1C(C(C=C2)C)CCC3CC(CC(=O)O3)O | ||
Mechanism of Action
| Targets/IC50/Ki |
HMG-CoA reductase
|
|---|---|
| In vitro |
Mevastatin is a cholesterol-lowering agent isolated from Penicillium citinium. It reduces cholesterol synthesis to 50% of control at 0.01 pg/mL (26 nM). This compound is structurally similar to the HMG, a substituent of the endogenous substrate of HMG-CoA reductase. It is a prodrug that is activated in vivo via hydrolysis of the lactone ring. The hydrolyzed lactone ring mimics the tetrahedral intermediate produced by the reductase allowing the agent to bind with 10,000 times greater affinity than its natural substrate. The bicyclic portion of this chemical binds to the coenzyme A portion of the active site. It increases levels of eNOS mRNA and protein, reduces infarct size, and improves neurological deficits in a dose- and time-dependent manner. |
| In vivo |
At doses of 5 and 20 mg/kg, mevastatin produces reduction of serum cholesterol levels at 3 hours after oral administration. It lowers the levels of serum cholesterol by approximately 30 % at a dose of 20 mg/kg. This compound lowers hepatic production of cholesterol by competitively inhibiting HMG-CoA reductase. Cholesterol levels are reduced only after 28 days of treatment and does not correlate with infarct reduction. Baseline absolute cerebral blood flow is 30% higher after 14-day high-dose treatment. |
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































