- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Fluticasone propionate Glucocorticoid Receptor agonist
Cat.No.S1992
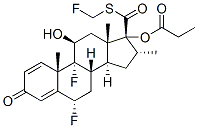
Chemical Structure
Molecular Weight: 500.57
Jump to
Quality Control
| Related Targets | Adrenergic Receptor Estrogen/progestogen Receptor GPR Androgen Receptor ACE RAAS Progesterone Receptor Opioid Receptor PGES THR |
|---|---|
| Other Glucocorticoid Receptor Products | AL082D06 (20S)-Protopanaxatriol Corticosterone Glucocorticoid Receptor Antibody [D9N22] Cortodoxone |
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(199.77 mM)
Ethanol : 5 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 500.57 | Formula | C25H31F3O5S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 80474-14-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | CCI-187881 | Smiles | CCC(=O)OC1(C(CC2C1(CC(C3(C2CC(C4=CC(=O)C=CC43C)F)F)O)C)C)C(=O)SCF | ||
Mechanism of Action
| Targets/IC50/Ki |
Glucocorticoid receptor
|
|---|---|
| In vitro |
Fluticasone propionate (1 pM) inhibits the constitutive and TGF-beta-induced expression of alpha-SMA in human lung myofibroblasts. This compound blocks the TNF-alpha-induced nuclear translocation of the pro-inflammatory transcription factor NF-kappaB in human lung myofibroblasts. It inhibits in lung myofibroblasts, at a very early stage of differentiation, the activation of Janus kinase/STAT pathways induced by IL-13 (tyrosine kinase 2, STAT1, STAT3, STAT6, mitogen-activated protein kinase). This chemical still displays a potential anti-inflammatory activity even if it only inhibits tyrosine kinase 2 phosphorylation in mildly or fully differentiated myofibroblastic cultures. It inhibits constitutive and TGF-beta-induced expression of alpha-smooth muscle actin, the main marker of myofibroblastic differentiation, both in very early and in mild differentiated myofibroblasts. This compound displays an additional powerful anti-inflammatory effect, decreasing nuclear translocation of NF-kappaB independent of the degree of myofibroblastic differentiation. It inhibits allergen-induced T-cell proliferation, expression of IL-3, IL-5 and GM-CSF mRNA, and secretion of the corresponding proteins in a concentration-dependent fashion. This chemical has the potential markedly to inhibit allergen-induced T-cell production of asthma-relevant cytokines.
|
| In vivo |
Fluticasone propionate administrated after induction of a severe heaves exacerbation results in complete resolution of clinical signs, normalization of pulmonary function tests, and significant decrease in bronchoalveolar lavage (BAL) neutrophilia in horse.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06290102 | Recruiting | Asthma |
Teva Branded Pharmaceutical Products R&D Inc. |
May 2 2024 | Phase 1 |
| NCT02630121 | Recruiting | Sleep Apnea|Chronic Nasal Congestion |
University of South Florida |
April 2023 | Phase 4 |
| NCT05608681 | Recruiting | Eosinophilic Esophagitis |
Eupraxia Pharmaceuticals Inc. |
March 31 2023 | Phase 1|Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































