research use only
APD668 GPR agonist
Cat.No.S6746
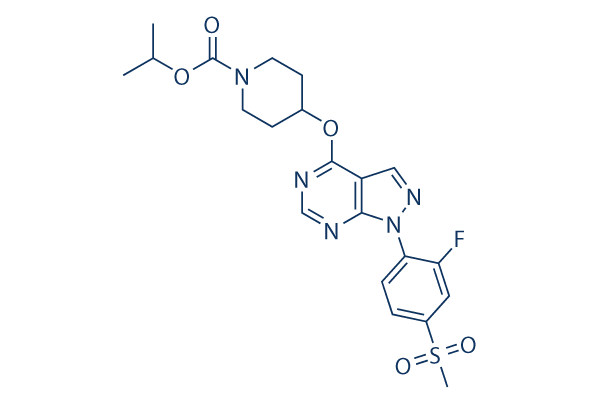
Chemical Structure
Molecular Weight: 477.51
Quality Control
Solubility
|
In vitro |
DMSO
: 96 mg/mL
(201.04 mM)
Ethanol : 2 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 477.51 | Formula | C21H24FN5O5S |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 832714-46-2 | -- | Storage of Stock Solutions |
|
|
| Synonyms | JNJ28630368 | Smiles | CC(C)OC(=O)N1CCC(CC1)OC2=NC=NC3=C2C=NN3C4=C(C=C(C=C4)S(=O)(=O)C)F | ||
Mechanism of Action
| Targets/IC50/Ki |
human GPR119
2.7 nM(EC50)
rat GPR119
33 nM(EC50)
|
|---|---|
| In vitro |
APD668 is shown to increase adenylate cyclase activation in HEK293 cells transfected with human GPR119 (but not in non-transfected cells) in a concentration-dependent manner with an EC50 of 23 nM. This compound also enhanced insulin release from both rat and human isolated pancreatic islets in a glucose-dependent manner. In a standard panel of around 80 known receptors and ion channels, it did not show any binding in excess of 50% of control to any other proteins at concentrations up to 10 μM. |
| In vivo |
Chronic treatment with APD668 showed that blood glucose and glycated hemoglobin (HbA1c) levels could be significantly reduced in Zucker Diabetic Fatty (ZDF) rats over several weeks of dosing. This compound is highly bound to plasma proteins of male and female cynomolgus monkeys and humans (P99%), but was less extensively bound to male (93.0%) and female (96.6%) rats. In pharmacokinetic assessments across multiple species using single oral doses of this chemical, absorption was rapid to moderate (Tmax 62 h) in mice, Sprague-Dawley (SD) rats, and monkeys, but slower in dogs (Tmax = 6 h) and showed a dose-dependent increase in rats and monkeys. In general, exposure was dose-dependent at lower doses and appeared to plateau at doses greater than 300 mg/kg. Exposure was greater in female rats compared with males. Absolute oral bioavailability was moderate to good in mice, rats, and monkeys (44–79%), but was lower in dogs (22%). The volume of distribution (Vdss) values were somewhat variable ranging from 0.1 L/kg in monkey to 2.6 L/kg in rats. Elimination, based on mean T1/2 after intravenous (iv) dosing, was rapid to moderate in mice, rats, dogs, and monkeys (0.8-3.9 h). The pharmacokinetic profile of this compound in Zucker fa/fa rats was somewhat different from that in SD rats. After oral administration, the tmax and T1/2 were longer, and the AUC and the oral bioavailability were greater in the Zucker compared with the SD rats. Following iv administration, the Zucker rats also had larger AUC values, longer T1/2 values and greater Vdss values. |
References |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































