research use only
Triamcinolone Acetonide Glucocorticoid Receptor agonist
Cat.No.S1628
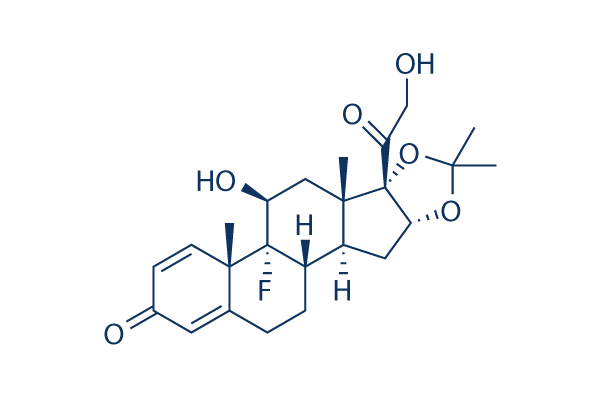
Chemical Structure
Molecular Weight: 434.5
Quality Control
| Related Targets | Dehydrogenase HSP Transferase P450 (e.g. CYP17) PDE phosphatase PPAR Vitamin Carbohydrate Metabolism Mitochondrial Metabolism |
|---|---|
| Other Glucocorticoid Receptor Inhibitors | Corticosterone AL082D06 (20S)-Protopanaxatriol Cortodoxone |
Solubility
|
In vitro |
DMSO
: 86 mg/mL
(197.92 mM)
Ethanol : 17 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 434.5 | Formula | C24H31FO6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 76-25-5 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | N/A | Smiles | CC1(OC2CC3C4CCC5=CC(=O)C=CC5(C4(C(CC3(C2(O1)C(=O)CO)C)O)F)C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
Glucocorticoid receptor
|
|---|---|
| In vitro |
Triamcinolone Acetonide significantly decreases the paracellular permeability of ECV304 cells and down-regulated ICAM-1 expression, consistent with immunocytochemical observations. This compound reverses the osmotic swelling of glial cells in retinas of the rat that is observed under various experimental conditions: in retinas isolated at 3 days after transient retinal ischemia, in retinas of eyes with lipopolysaccharide-induced ocular inflammation, and in control retinas in the presence of Ba2+ (1 mM), H2O2 (200 mM), arachidonic acid (10 mM), or prostaglandin E2 (30 nM). It reduces GAG synthesis compared to control and IL-1 alone. This chemical increases GAG degradation. It increases media GAG content compared to control and IL-1 explants.
|
| In vivo |
Triamcinolone Acetonide (TAC), a synthetic glucocorticoid, induces cleft palate resulting from poor development of palatal shelves in mice. This compound inhibits the proliferation of mesenchymal cells and affects the differentiation of MEE cells into stratified squamous epithelia in the palatal shelves of rat embryos. It reduces lameness, edema, and concentration of synovial fluid protein after the second LPS injection in horse. This chemical also induces higher WBC counts and mepivacaine concentrations in synovial fluid, compared with results for Mepivacaine alone.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT04658836 | Completed | Vestibular Schwannoma |
Medical University of Vienna |
February 1 2020 | Phase 1 |
| NCT03378076 | Completed | Bilateral Knee Osteoarthritis |
Pacira Pharmaceuticals Inc |
December 6 2017 | Phase 2 |
| NCT03382262 | Completed | Osteoarthritis of the Shoulder|Osteoarthritis of the Hip |
Pacira Pharmaceuticals Inc |
December 18 2017 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































