research use only
Tenofovir hydrate Reverse Transcriptase inhibitor
Cat.No.S4879
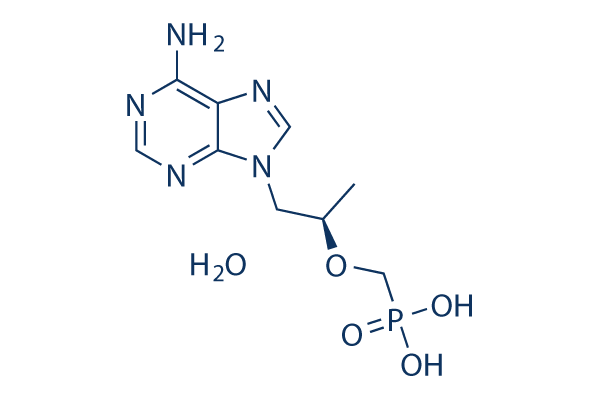
Chemical Structure
Molecular Weight: 305.23
Quality Control
| Related Targets | Integrase Bacterial Antibiotics Anti-infection Fungal Antiviral COVID-19 Parasite HIV HCV Protease |
|---|---|
| Other Reverse Transcriptase Inhibitors | Dapivirine (TMC120) Fangchinoline 3'-Fluoro-3'-deoxythymidine (Alovudine) Salicylanilide Bifendate Ulonivirine Lersivirine (UK-453061) 4-Chloro-2-(trifluoroacetyl)aniline hydrochloride |
Solubility
|
In vitro |
Water : 10 mg/mL
DMSO
: Insoluble
Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 305.23 | Formula | C9H16N5O5P |
Storage (From the date of receipt) | 3 years -20°C powder |
|---|---|---|---|---|---|
| CAS No. | 206184-49-8 | -- | Storage of Stock Solutions |
|
|
| Synonyms | GS-1278 hydrate | Smiles | O.CC(C[N]1C=NC2=C(N)N=CN=C12)OC[P](O)(O)=O | ||
Mechanism of Action
| In vitro |
Tenofovir reduces the viral cytopathic effect of HIV-1(IIIB), HIV-2(ROD) and HIV(EHO) with EC50 of 1.15 μg/mL, 1.12 μg/mL and 1.05 μg/mL in MT-4 cells. Tenofovir also reduces the viral cytopathic effect of SIV(mac251) , SIV(B670) ,SHIV(89.6) and SHIV(RTSHIV). Tenofovir is uniquely active against multinucleoside-resistant HIV expressing the Q151M mutation, but shows reduced susceptibility to the T69S insertion mutations. Tenofovir inhibits hepatitis B virus (HBV) activity in HepG2 2.2.15, HepAD38 and HepAD79 cells. Tenofovir (4 μM) completely inhibits the growth of HIVIIIB in MT-2 cells. Tenofovir inhibits synthesis of negative strand strong-stop DNA with IC50 of 9 µM for wild-type RT, 6 µM for M184V RT and 50 µM for K65R RT. |
|---|---|
| In vivo |
Tenofovir (30 mg/kg) completely prevents SIV infection in all macaques without toxicity. Tenofovir treatment reduces plasma viral RNA levels to undetectable, with parallel decreases in the infectivity of plasma and infectious cells in peripheral blood mononuclear cells and cerebrospinal fluid (CSF) and stabilization of CD4+ T-cell numbers. Tenofovir (30 mg/kg, s.c.) completely abrogates HIV infection via intravaginal exposure in pig-tailed macaques. |
References |
|
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































