- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Telaprevir HCV Protease inhibitor
Cat.No.S1538
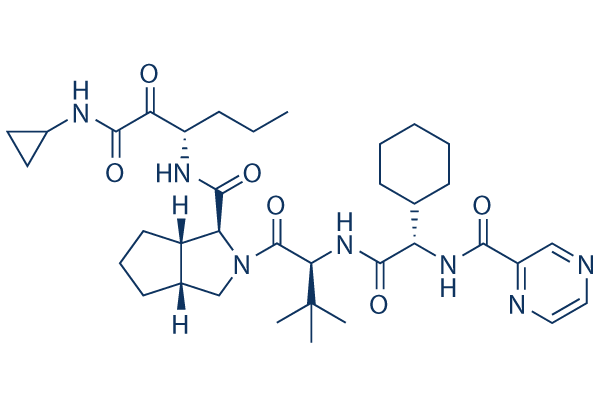
Chemical Structure
Molecular Weight: 679.85
Jump to
Quality Control
Batch:
Purity:
99.82%
99.82
| Related Targets | HDAC Caspase Proteasome Secretase MMP Cysteine Protease DPP Tyrosinase HIV Protease Serine Protease |
|---|---|
| Other HCV Protease Inhibitors | Lomibuvir (VX-222) Danoprevir Asunaprevir PSI-6206 (GS-331007) 2'-C-Methylcytidine Tegobuvir Tizoxanide Herba taxilli Extract Mecarbinate |
Solubility
|
In vitro |
DMSO
: 136 mg/mL
(200.04 mM)
Water : Insoluble Ethanol : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 679.85 | Formula | C36H53N7O6 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 402957-28-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | LY-570310, MP-424,VX-950 | Smiles | CCCC(C(=O)C(=O)NC1CC1)NC(=O)C2C3CCCC3CN2C(=O)C(C(C)(C)C)NC(=O)C(C4CCCCC4)NC(=O)C5=NC=CN=C5 | ||
Mechanism of Action
| Features |
Telaprevir is a covalent, reversible inhibitor of the NS3-4A protease (unlike BILN 2061which is a noncovalent inhibitor), with a slow-binding and slow-dissociation mechanism.
|
|---|---|
| Targets/IC50/Ki |
HCV NS3-4A serine protease
0.35 μM
|
| In vitro |
Telaprevir inhibits the hepatitis C virus NS3-4A serine protease, leading to the block of viral polyprotein processing and subsequently decrease of viral RNA replication, total HCV RNA levels and protein levels in the Con1 (genotype 1b) subgenomic HCV replicon cells in a time- and dose-dependent manner. This compound displays a significant time-dependent increase in inhibitory effect on the replication of HCV RNA with IC50 values of 0.574 μM, 0.488 μM, 0.210 μM and 0.139 μM for 24, 48, 72 and 120 hours incubation, respectively. It displays an average IC50 of 0.354 μM and an average IC90 of 0.830 μM, respectively, from three independent experiments using the 48 hours incubation. This chemical has no significant cytotoxicity to HCV replicon cells, parental Huh-7 and HepG2 cells after 48 hours incubation. It (17.5 μM) completely eradicates HCV RNA from replicon cells after 13 days incubation without rebound after this compound is withdrawn. It displays an additive to moderate synergistic effect on reduction of HCV RNA replication and suppression of resistance mutations without significant increase in cytotoxicity when in combination with IFN-α, compared to treatment with each agent alone.
|
| Kinase Assay |
Determination of anti-HCV activity
|
|
Stable Huh-7 cells containing the self-replicating, subgenomic HCV replicon, which is identical in sequence to the I377neo/NS3-3'/wt replicon are used for anti-HCV assays. Replicon cells are incubated at 37 °C for the indicated period of time with this compound serially diluted in DMEM plus 2% FBS and 0.5% dimethyl sulfoxide (DMSO). Total cellular RNA is extracted using an RNeasy-96 kit, and the copy number of HCV RNA is determined using a quantitative RTPCR (QRT-PCR) assay for the assessment of 50% inhibitory concentration (IC50
|
|
| In vivo |
Oral administration of Telaprevir reduces HCV protease-dependent cleavage and subsequent secretion of SEAP from the liver into the blood in the mice model to 18.7% and 18.4% at dosage of 10 and 25 mg/kg, respectively. Administration of this compound at 200 mg/kg for 1 week results in a 1.9 log reduction of HCV RNA in genotype 1b HCV-infected human hepatocyte chimeric mice, and when treatment in combination with MK-0608 (50 mg/kg) for 4 weeks, viruses are eliminated from mice.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT02881034 | Completed | Hepatitis C |
Hospices Civils de Lyon |
February 2014 | -- |
| NCT01994486 | Completed | Hepatitis C Chronic |
University of Florida|Vertex Pharmaceuticals Incorporated |
December 2013 | Phase 2 |
| NCT01980290 | Completed | Hepatitis Chronic |
Janssen Pharmaceutica N.V. Belgium |
May 2013 | -- |
| NCT01841502 | Terminated | Hepatitis C Infection|Depression |
Radboud University Medical Center|Janssen LP |
May 2013 | Phase 2 |
| NCT01766167 | Completed | Chronic Hepatitis C |
Mitsubishi Tanabe Pharma Corporation |
February 2013 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































