- Inhibitors
- Antibodies
- Compound Libraries
- New Products
- Contact Us
research use only
Asunaprevir HCV Protease inhibitor
Cat.No.S4935
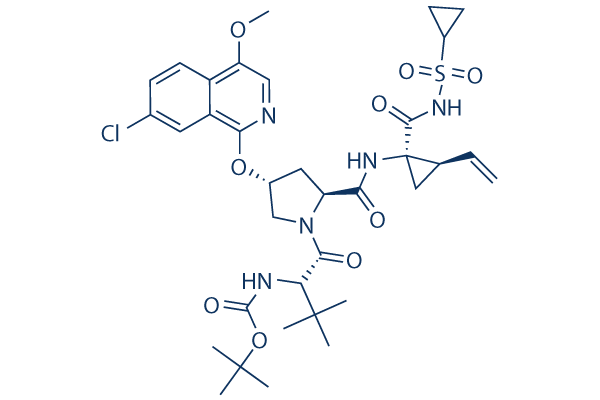
Chemical Structure
Molecular Weight: 748.29
Jump to
Quality Control
Batch:
Purity:
99.99%
99.99
| Related Targets | HDAC Caspase Proteasome Secretase MMP Cysteine Protease DPP Tyrosinase HIV Protease Serine Protease |
|---|---|
| Other HCV Protease Products | Lomibuvir (VX-222) Danoprevir PSI-6206 (GS-331007) 2'-C-Methylcytidine Tegobuvir Tizoxanide Herba taxilli Extract Mecarbinate |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| genotype 1b con1 replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 4a ED43 NS3 protease in genotype 1b con1 replicon cells by luciferase reporter gene assay, EC50=0.0017μM | 27564532 | |||
| Huh7.5 cells | Antiviral assay | Antiviral activity against HCV genotype 1b Con1 expressing NS3 protease infected in human Huh7.5 cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.003μM | 27564532 | |||
| HCV replicon cells | Antiviral assay | Antiviral activity against HCV genotype 1a H77 in HCV replicon cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.004μM | 27564532 | |||
| HCV replicon cells | Antiviral assay | Antiviral activity against HCV genotype 2a JHF-1 in HCV replicon cells assessed as reduction in viral RNA replication by luciferase reporter gene assay, EC50=0.217μM | 27564532 | |||
| genotype 2a replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 2b HC-J8 NS3 protease in genotype 2a replicon cells by luciferase reporter gene assay, EC50=0.621μM | 27564532 | |||
| genotype 2a replicon cells | Function assay | Inhibition of recombinant full length HCV genotype 3a S52 NS3 protease in genotype 2a replicon cells by luciferase reporter gene assay, EC50=1.1μM | 27564532 | |||
| HuH7 cells | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells at >= 10 times antiviral EC50 after 3 days by luciferase reporter assay | 28430437 | ||
| HuH7 cells | Antiviral assay | 3 days | Antiviral activity against HCV genotype 1b infected in human HuH7 cells co-treated with daclatasvir after 3 days by luciferase reporter assay | 28430437 | ||
| HuH7 replicon cells | Function assay | 4 days | Inhibition of HCV genotype 1b Con1 NS3 protease infected in human HuH7 replicon cells assessed as reduction in viral replication after 4 days by luciferase reporter gene assay, EC50=0.0012μM | 29162454 | ||
| HuH7 replicon cells | Function assay | 4 days | Inhibition of HCV genotype 1a H77 NS3 protease infected in human HuH7 replicon cells assessed as reduction in viral replication after 4 days by luciferase reporter gene assay, EC50=0.004μM | 29162454 | ||
| HuH7.5 cells | Antiviral assay | 4 days | Antiviral activity against HCV genotype 1b Con1 infected in human HuH7.5 cells assessed as inhibition of viral replication after 4 days by luciferase reporter gene assay, EC50=0.006μM | 29456803 | ||
| Huh7.5 replicon cells | Antiviral assay | 4 days | Antiviral activity against HCV genotype 1b infected in human Huh7.5 replicon cells assessed as reduction in viral replication incubated for 4 days by luciferase reporter gene assay, EC50=0.006μM | 29650290 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(133.63 mM)
Ethanol : 100 mg/mL Water : Insoluble |
Molarity Calculator
Dilution Calculator
Molecular Weight Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
%
DMSO
%
%
Tween 80
%
ddH2O
%
DMSO
+
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 748.29 | Formula | C35H46ClN5O9S |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 630420-16-5 | -- | Storage of Stock Solutions |
|
|
| Synonyms | BMS-650032 | Smiles | CC(C)(C)C(C(=O)N1CC(CC1C(=O)NC2(CC2C=C)C(=O)NS(=O)(=O)C3CC3)OC4=NC=C(C5=C4C=C(C=C5)Cl)OC)NC(=O)OC(C)(C)C | ||
Mechanism of Action
| Targets/IC50/Ki |
1b (J4L6S)
0.3 nM
1a (H77)
0.7 nM
6a (HK-6A)
0.9 nM
4a (ED43)
1.6 nM
5a (SA13)
1.7 nM
2a (HC-J6)
15 nM
2b (HC-J8)
78 nM
|
|---|---|
| In vitro |
Asunaprevir (ASV) competitively binds to the NS3/4A protease complex, with Ki values of 0.4 and 0.24 nM against recombinant enzymes representing genotypes 1a (H77) and 1b (J4L6S), respectively. This compound is high selective without any significant activity against the closely related GB virus-B NS3 protease and a panel of human serine or cysteine proteases. In cell culture, it inhibits replication of HCV replicons representing genotypes 1 and 4, with 50% effective concentrations (EC50s) ranging from 1 to 4 nM, and has weaker activity against genotypes 2 and 3 (EC50, 67-1162 nM). Selectivity is again demonstrated by the absence of activity (EC50, >12 μM) against a panel of other RNA viruses.
|
| In vivo |
Plasma and tissue exposures in vivo in several animal species indicate that this compound displays a hepatotropic disposition (liver-to-plasma ratios ranging from 40- to 359-fold across species). Twenty-four hours postdose, liver exposures across all species tested are ≥110-fold above the inhibitor EC50s observed with HCV genotype-1 replicons.
|
References |
|
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03208322 | Withdrawn | Hepatitis C |
Bristol-Myers Squibb |
November 30 2018 | -- |
| NCT03004625 | Completed | Hepatitis C |
Kaohsiung Medical University Chung-Ho Memorial Hospital|Chang Gung Memorial Hospital|National Taiwan University Hospital|Taipei Veterans General Hospital Taiwan|China Medical University Hospital|National Cheng-Kung University Hospital |
November 2016 | Phase 3 |
| NCT02865369 | Unknown status | Chronic Hepatitis C |
Sang Gyune Kim|Seoul National University Boramae Hospital|Severance Hospital|Inha University Hospital|Korea University|Gachon University Gil Medical Center|Hanyang University Seoul Hospital|Ewha Womans University Mokdong Hospital|Bristol-Myers Squibb|Soonchunhyang University Hospital |
September 2016 | -- |
| NCT02580474 | Completed | Hepatitis C |
Myeong Jun Song|Bristol-Myers Squibb|Soonchunhyang University Hospital|Dankook University|Chungnam National University Hospital|Konyang University Hospital|Eulji University Hospital|Saint Vincent''s Hospital Korea|Konkuk University Hospital|Cheongju St. Mary''s Hospital Cheongju Korea|Severance Hospital|Korea University Guro Hospital|Eulji General Hospital|The Catholic University of Korea |
February 2016 | Phase 4 |
| NCT02496078 | Completed | Hepatitis C |
Bristol-Myers Squibb |
August 2015 | Phase 3 |
| NCT02309450 | Withdrawn | Hepatitis C Virus Genotype 4 Infection |
ANRS Emerging Infectious Diseases|Bristol-Myers Squibb |
December 2014 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.






































