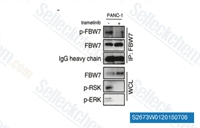
| S2673 |
Trametinib (GSK1120212)
|
Trametinib (GSK1120212, JTP-74057) is a highly specific and potent MEK1/2 inhibitor with IC50 of 0.92 nM/1.8 nM in cell-free assays, and it does not inhibit the kinase activities of c-Raf, B-Raf, ERK1/2. This compound activates autophagy and induces apoptosis.
|
-
Cancer Cell, 2025, S1535-6108(25)00271-5
-
Signal Transduct Target Ther, 2025, 10(1):161
-
Signal Transduct Target Ther, 2025, 10(1):299
|
|
| S1036 |
PD0325901 (Mirdametinib)
|
Mirdametinib (PD0325901) is a selective and non ATP-competitive MEK inhibitor with IC50 of 0.33 nM in cell-free assays, roughly 500-fold more potent than CI-1040 on phosphorylation of ERK1 and ERK2. Phase 2.
|
-
Nature, 2025, 10.1038/s41586-025-09328-w
-
Nature, 2025, 10.1038/s41586-025-09571-1
-
Cell, 2025, S0092-8674(25)00807-4
|

|
| S1008 |
AZD6244 (Selumetinib)
|
Selumetinib (AZD6244, ARRY-142886) is a potent, highly selective MEK inhibitor with IC50 of 14 nM for MEK1 and Kd value of 530 nM for MEK2. It also inhibits ERK1/2 phosphorylation with IC50 of 10 nM, no inhibition to p38α, MKK6, EGFR, ErbB2, ERK2, B-Raf, etc. Selumetinib suppresses cell proliferation, migration and trigger apoptosis. Phase 3.
|
-
Nat Commun, 2025, 16(1):4884
-
Cell Rep Med, 2025, S2666-3791(25)00102-8
-
Cell Rep, 2025, 44(6):115774
|

|
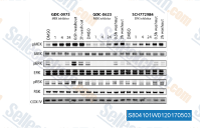
| S8041 |
Cobimetinib (GDC-0973)
|
Cobimetinib (GDC-0973, RG7420) is a potent and highly selective MEK1 inhibitor with IC50 of 4.2 nM, showing no significant inhibition when tested against a panel of more than 100 serine-threonine and tyrosine kinases. This compound induces apoptosis. Phase 3.
|
-
Cancer Cell, 2025, 43(3):482-502.e9
-
Hepatology, 2025, 10.1097/HEP.0000000000001439
-
Cancer Res, 2025, 10.1158/0008-5472.CAN-24-3819
|
|
| S7170 |
Avutometinib (Ro5126766, CH5126766)
|
Avutometinib(RO5126766,CH5126766,VS 6766, CKI-27, R-7304, RG-7304) is a dual RAF/MEK inhibitor with IC50 of 8.2 nM,19 nM, 56 nM, and 160 nM for BRAF V600E, BRAF, CRAF, and MEK1, respectively. Phase 1.
|
-
Cancer Chemother Pharmacol, 2025, 95(1):78
-
Nat Biomed Eng, 2024, 10.1038/s41551-024-01273-9
-
Cell Rep Med, 2024, 5(11):101818
|
|
| S1102 |
U0126-EtOH
|
U0126-EtOH is a highly selective inhibitor of MEK1/2 with IC50 of 0.07 μM/0.06 μM in cell-free assays, 100-fold higher affinity for ΔN3-S218E/S222D MEK than PD98059. U0126 inhibits autophagy and mitophagy with antiviral activity.
|
-
Nat Commun, 2025, 16(1):2192
-
Nat Commun, 2025, 16(1):7156
-
Adv Sci (Weinh), 2025, 12(44):e11726
|

|
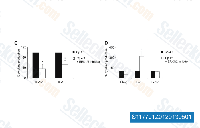
| S1177 |
PD98059
|
PD98059 is a non-ATP competitive MEK inhibitor with IC50 of 2 μM in a cell-free assay, specifically inhibits MEK-1-mediated activation of MAPK; does not directly inhibit ERK1 or ERK2. This compound is a ligand for the aryl hydrocarbon receptor (AHR) and functions as an AHR antagonist.
|
-
Nat Commun, 2025, 16(1):212
-
Adv Sci (Weinh), 2025, 12(28):e2502634
-
Theranostics, 2025, 15(6):2624-2648
|
|
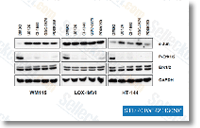
| S1020 |
PD184352 (CI-1040)
|
PD184352 (CI-1040) is an ATP non-competitive MEK1/2 inhibitor with IC50 of 17 nM in cell-based assays, 100-fold more selective for MEK1/2 than MEK5. This compound selectively induces apoptosis.
|
-
Cell Host Microbe, 2025, 33(4):512-528.e7
-
Int J Mol Sci, 2025, 26(8)3536
-
Front Cell Dev Biol, 2025, 13:1601887
|
|
| S7007 |
Binimetinib (MEK162)
|
Binimetinib (MEK162, ARRY-162, ARRY-438162) is a potent inhibitor of MEK1/2 with IC50 of 12 nM in a cell-free assay. It induces G1 cell cycle arrest and apoptosis in human NSCLC cell lines and also triggers autophagy. Phase 3.
|
-
Cell Rep Med, 2025, 6(2):101943
-
Cell Syst, 2025, 16(3):101229
-
Biochem Pharmacol, 2025, 235:116842
|

|
| S1531 |
BIX 02189
|
BIX02189 is a selective inhibitor of MEK5 with IC50 of 1.5 nM, also inhibits ERK5 catalytic activity with IC50 of 59 nM in cell-free assays, and does not inhibit closely related kinases MEK1, MEK2, ERK2, and JNK2.
|
-
PLoS One, 2024, 19(1):e0295629
-
Nat Commun, 2023, 10.1038/s41467-023-43369-x
-
Exp Mol Med, 2023, 55(6):1247-1257
|

|














































