research use only
Cobimetinib (GDC-0973) MEK1 inhibitor
Cat.No.S8041
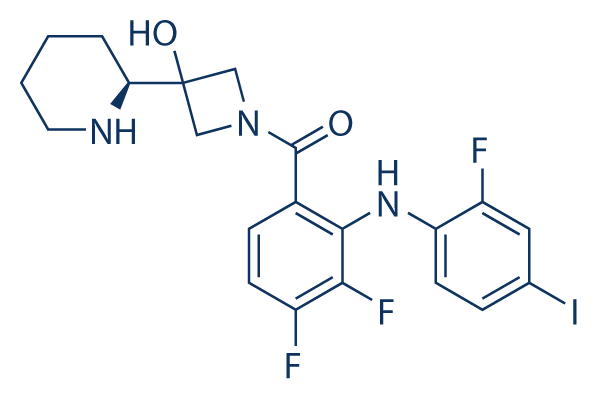
Chemical Structure
Molecular Weight: 531.31
Quality Control
| Related Targets | ERK p38 MAPK Raf JNK Ras KRas S6 Kinase MAP4K TAK1 Mixed Lineage Kinase |
|---|---|
| Other MEK Inhibitors | PD0325901 (Mirdametinib) U0126-EtOH PD 98059 PD184352 (CI-1040) BIX 02189 Pimasertib (AS-703026) Refametinib (RDEA119) TAK-733 AZD8330 BIX 02188 |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| human COLO205 cells | Proliferation assay | Antiproliferative activity against human COLO205 cells expressing B-raf V600E mutant, IC50=8 nM | 22315332 | |||
| human COLO205 cells | Function assay | Inhibition of B-raf V600E mutant in human COLO205 cells assessed as reduction of ERK1/ERK2 phosphorylation, IC50=1.8 nM | 22315332 | |||
| human MDA-MB-231T cells | Function assay | 1 h | Inhibition of MEK-mediated ERK T202/Y204 phosphorylation in human MDA-MB-231T cells after 1 hr by immunoblotting, IC50=0.2 nM | 24900486 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(188.21 mM)
Ethanol : 47 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 531.31 | Formula | C21H21F3IN3O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 934660-93-2 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | RG7420,XL518 | Smiles | C1CCNC(C1)C2(CN(C2)C(=O)C3=C(C(=C(C=C3)F)F)NC4=C(C=C(C=C4)I)F)O | ||
Mechanism of Action
| Targets/IC50/Ki |
MEK1
(Cell-free assay) 4.2 nM
|
|---|---|
| In vitro |
Cobimetinib (GDC-0973) shows strong activity on cell growth inhibtion in a broad panel of tumor types, particularly in BRAF or KRAS mutant cancer cell lines. In combination with GDC-0941, it results in reduced viability, pathway inhibition, and increased apoptosis in 888MEL and A2058 cells.
|
| In vivo |
In mice bearing BRAFV600E and KRAS mutant tumors, Cobimetinib (GDC-0973) (10 mg/kg, p.o.) produces antitumor efficacy, and the combination of this compound and GDC-0941 show improved efficacy. In mice bearing drug-resistant A375 xenografts, the combination of it and GDC-0941 induces decreased levels of hexokinase II, c-RAF, Ksr and p-MEK protein. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pERK / ERK / MCL-1 / p-MCL1 / BIM / cleaved PARP p-c-RAF / c-RAF / p-MEK / MEK |
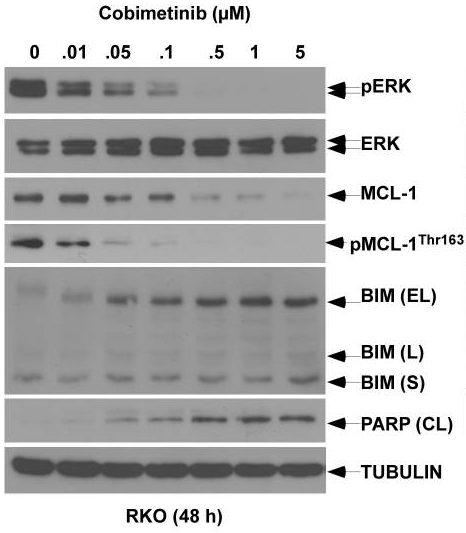
|
27765849 |
| Growth inhibition assay | Cell viability |
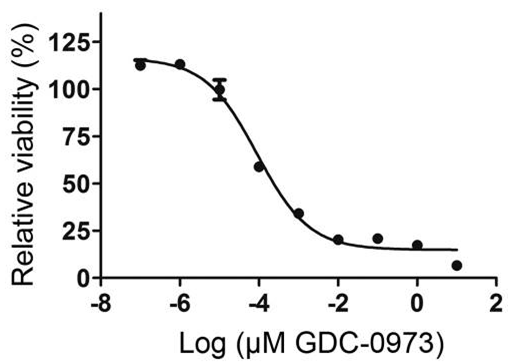
|
28098866 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05756556 | Suspended | Melanoma|Malignant Melanoma |
ImmVira Pharma Co. Ltd |
June 30 2024 | Phase 2 |
| NCT05768178 | Recruiting | Solid Tumor|Haematological Malignancy|Melanoma|Thyroid Cancer Papillary|Ovarian Neoplasms|Colorectal Neoplasms|Laryngeal Neoplasms|Carcinoma Non-Small-Cell Lung|Glioma|Multiple Myeloma|Erdheim-Chester Disease|Thyroid Carcinoma Anaplastic |
Cancer Research UK|University of Manchester|University of Birmingham|Royal Marsden NHS Foundation Trust|Hoffmann-La Roche |
March 1 2023 | Phase 2|Phase 3 |
| NCT04835805 | Active not recruiting | Melanoma |
Genentech Inc. |
May 13 2021 | Phase 1 |
| NCT04109456 | Recruiting | Metastatic Melanoma |
InxMed (Shanghai) Co. Ltd. |
March 16 2020 | Phase 1 |
| NCT04007848 | Completed | Disease or R Group Histiocytoses |
Assistance Publique - Hôpitaux de Paris |
July 25 2019 | Phase 3 |
| NCT03695380 | Completed | OVARIAN CANCER |
Hoffmann-La Roche |
January 9 2019 | Phase 1 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
How to reconstitute it for in vivo studies?
Answer:
It can be dissolved in 5% DMSO/30% PEG 300/5% Tween 80/ddH2O at 5 mg/ml clearly and is suitable for both oral gavage and injection.






































