- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Dinaciclib
Synonyms: SCH727965, PS-095760
Home Cell Cycle CDK inhibitor - Caspase activator Dinaciclib
For research use only.
Dinaciclib is a novel and potent CDK inhibitor for CDK2, CDK5, CDK1 and CDK9 with IC50 of 1 nM, 1 nM, 3 nM and 4 nM in cell-free assays, respectively. It also blocks thymidine (dThd) DNA incorporation. Dinaciclib induces apoptosis through the activation of caspases 8 and 9. Phase 3.
-chemical-structure-s2768.gif)
Dinaciclib Chemical Structure
CAS No. 779353-01-4
Purity & Quality Control
Batch:
Purity:
99.99%
99.99
Products often used together with Dinaciclib
Dinaciclib and MK-2206 2HCl combination use dramatically blocks tumor growth and markedly reduces the number of metastatic lesions in the orthotopic Panc265/Panc253 models.
Dinaciclib and SCH772984 combination use results in a more significant reduction in tumor growth than either treatment alone in mice.
Rapamycin administration to mice treated with LPS greatly improves gut inflammation and oxidative stress, while 3-methyladenine had the opposite effect.
Dinaciclib Related Products
| Related Targets | CDK1 CDK2 CDK3 CDK4 CDK5 CDK6 CDK7 CDK9 CLK Cdc CDK8 CDK12 CDK13 CDK19 CDK11 CDK/cyclin complexes | Click to Expand |
|---|---|---|
| Related Products | Ro-3306 Roscovitine Flavopiridol (Alvocidib) SNS-032 (BMS-387032) Flavopiridol (Alvocidib) HCl AT7519 LDC000067 PHA-767491 HCl THZ1 2HCl XL413 JNJ-7706621 AZD5438 Purvalanol A Milciclib BS-181 HCl K03861 (AUZ454) PHA-793887 BMS-265246 AT7519 HCl AZD4573 | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library PI3K/Akt Inhibitor Library MAPK Inhibitor Library DNA Damage/DNA Repair compound Library Cell Cycle compound library | Click to Expand |
Signaling Pathway
Cell Data
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| CA46 | Apoptosis Assay | 100 nM | 24 h | induces cell cycle arrest | 25289887 | |
| Kasumi-1 | Apoptosis Assay | 100 nM | 24 h | induces cell cycle arrest | 25289887 | |
| U937 | Function Assay | 2/5/10 nM | 3 h | blocks induction of XBP-1s and downstream targets | 24362465 | |
| 8226 | Function Assay | 2/5/10 nM | 4 h | blocks induction of XBP-1s and downstream targets | 24362465 | |
| H929 | Function Assay | 2/5/10 nM | 4 h | blocks induction of XBP-1s and downstream targets | 24362465 | |
| K562 | Function Assay | 1.5/3/8 nM | 6 h | blocks induction of XBP-1s and downstream targets | 24362465 | |
| BaF3/Bcr-abl | Function Assay | 1.5/3/8 nM | 6 h | blocks induction of XBP-1s and downstream targets | 24362465 | |
| U937 | Function Assay | 2/10 nM | 3 h | blocks induction of XBP-1s and downstream targets | 24362465 | |
| 1205Lu | Growth Inhibition Assay | 10/30 nM | 72 h | inhibits cell growth and survival | 23527225 | |
| WM1366 | Growth Inhibition Assay | 10/30 nM | 72 h | inhibits cell growth and survival | 23527225 | |
| RD | Growth Inhibition Assay | IC50=8.2 nM | 22315240 | |||
| Rh41 | Growth Inhibition Assay | IC50=10.5 nM | 22315240 | |||
| Rh18 | Growth Inhibition Assay | IC50=10.5 nM | 22315240 | |||
| Rh30 | Growth Inhibition Assay | IC50=9 nM | 22315240 | |||
| BT-12 | Growth Inhibition Assay | IC50=8.5 nM | 22315240 | |||
| CHLA-266 | Growth Inhibition Assay | IC50=7.3 nM | 22315240 | |||
| TC-71 | Growth Inhibition Assay | IC50=3.9 nM | 22315240 | |||
| CHLA-9 | Growth Inhibition Assay | IC50=8 nM | 22315240 | |||
| CHLA-10 | Growth Inhibition Assay | IC50=6.3 nM | 22315240 | |||
| CHLA-258 | Growth Inhibition Assay | IC50=9.9 nM | 22315240 | |||
| GBM2 | Growth Inhibition Assay | IC50=6.5 nM | 22315240 | |||
| NB-1643 | Growth Inhibition Assay | IC50=3.3 nM | 22315240 | |||
| NB-EBc1 | Growth Inhibition Assay | IC50=7 nM | 22315240 | |||
| CHLA-90 | Growth Inhibition Assay | IC50=7.5 nM | 22315240 | |||
| CHLA-136 | Growth Inhibition Assay | IC50=9.8 nM | 22315240 | |||
| NALM-6 | Growth Inhibition Assay | IC50=4.6 nM | 22315240 | |||
| COG-LL-317 | Growth Inhibition Assay | IC50=6.5 nM | 22315240 | |||
| RS4;11 | Growth Inhibition Assay | IC50=5.1 nM | 22315240 | |||
| MOLT-4 | Growth Inhibition Assay | IC50=9.3 nM | 22315240 | |||
| CCRF-CEM | Growth Inhibition Assay | IC50=5.6 nM | 22315240 | |||
| Kasumi-1 | Growth Inhibition Assay | IC50=4.5 nM | 22315240 | |||
| Karpas-299 | Growth Inhibition Assay | IC50=3.9 nM | 22315240 | |||
| Ramos-RA1 | Growth Inhibition Assay | IC50=7.9 nM | 22315240 | |||
| MIAPaCa-2 | Growth Inhibition Assay | 72 h | GI50=10 nM | 21768779 | ||
| Pa20C | Growth Inhibition Assay | 72 h | GI50=20 nM | 21768779 | ||
| ML-1 | Apoptosis Assay | 1-1000 nM | 4 h | induces apoptosis slightly | 21768777 | |
| Cytotoxicity assay | MDA-MB-436 | 72 hrs | IC50 = 0.005 μM | 23600925 | ||
| Cytotoxicity assay | NCI-H929 | 72 hrs | IC50 = 0.005 μM | 23600925 | ||
| Cytotoxicity assay | MDA-MB-231 | 72 hrs | IC50 = 0.005 μM | 23600925 | ||
| Cytotoxicity assay | SK-ES-1 | 72 hrs | IC50 = 0.005 μM | 23600925 | ||
| Cytotoxicity assay | A673 | 72 hrs | IC50 = 0.005 μM | 23600925 | ||
| Cytotoxicity assay | SK-BR-3 | 72 hrs | IC50 = 0.005 μM | 23600925 | ||
| Cytotoxicity assay | MNNG-HOS | 72 hrs | IC50 = 0.0055 μM | 23600925 | ||
| Cytotoxicity assay | SK-UT-1 | 72 hrs | IC50 = 0.006 μM | 23600925 | ||
| Cytotoxicity assay | U266 | 72 hrs | IC50 = 0.006 μM | 23600925 | ||
| Cytotoxicity assay | RPMI18226 | 72 hrs | IC50 = 0.009 μM | 23600925 | ||
| Cytotoxicity assay | SW872 | 72 hrs | IC50 = 0.0095 μM | 23600925 | ||
| Cytotoxicity assay | T47D | 72 hrs | IC50 = 0.01 μM | 23600925 | ||
| Apoptosis assay | A673 | 24 hrs | EC50 = 0.011 μM | 23600925 | ||
| Cytotoxicity assay | MCF7 | 72 hrs | IC50 = 0.02 μM | 23600925 | ||
| Function assay | Sf9 | IC50 = 0.072 μM | 26741853 | |||
| Function assay | sf9 | IC50 = 0.002 μM | 26851505 | |||
| Function assay | Sf9 | 1 hr | IC50 = 0.001 μM | 27171036 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.001 μM | 27171036 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.001 μM | 27171036 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.001 μM | 27171036 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.003 μM | 27171036 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.003 μM | 27171036 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.004 μM | 27171036 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.004 μM | 27171036 | ||
| Function assay | Sf9 | 10 uM | IC50 = 0.004 μM | 29329658 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.001 μM | 29853338 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.001 μM | 29853338 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.003 μM | 29853338 | ||
| Function assay | Sf9 | 1 hr | IC50 = 0.004 μM | 29853338 | ||
| Antiproliferative assay | MOLM13 | 72 hrs | GI50 = 0.0033 μM | 30253346 | ||
| Antiproliferative assay | MEC1 | 72 hrs | GI50 = 0.0036 μM | 30253346 | ||
| Antiproliferative assay | MOLM14 | 72 hrs | GI50 = 0.0045 μM | 30253346 | ||
| Antiproliferative assay | COLO205 | 72 hrs | GI50 = 0.0068 μM | 30253346 | ||
| Antiproliferative assay | HL60 | 72 hrs | GI50 = 0.008 μM | 30253346 | ||
| Antiproliferative assay | Ramos | 72 hrs | GI50 = 0.0086 μM | 30253346 | ||
| Antiproliferative assay | GISTT1 | 72 hrs | GI50 = 0.0088 μM | 30253346 | ||
| Antiproliferative assay | U937 | 72 hrs | GI50 = 0.01 μM | 30253346 | ||
| Antiproliferative assay | A431 | 72 hrs | GI50 = 0.011 μM | 30253346 | ||
| Antiproliferative assay | SKM1 | 72 hrs | GI50 = 0.011 μM | 30253346 | ||
| Antiproliferative assay | MEC2 | 72 hrs | GI50 = 0.011 μM | 30253346 | ||
| Antiproliferative assay | A375 | 72 hrs | GI50 = 0.011 μM | 30253346 | ||
| Antiproliferative assay | OCI-AML3 | 72 hrs | GI50 = 0.013 μM | 30253346 | ||
| Antiproliferative assay | BE(2)-M17 | 72 hrs | GI50 = 0.021 μM | 30253346 | ||
| Antiproliferative assay | CHO | 72 hrs | GI50 = 0.16 μM | 30253346 | ||
| Function assay | NCI-H929 | 0.005 uM | 24 hrs | Inhibition of CDK2-mediated Rb phosphorylation at Ser 807/811 in human NCI-H929 cells at 0.005 uM after 24 hrs by immunoblotting analysis | 23600925 | |
| Function assay | A673 | 0.05 uM | 24 hrs | Inhibition of CDK2-mediated Rb phosphorylation at Ser 807/811 in human A673 cells at 0.05 uM after 24 hrs by immunoblotting analysis | 23600925 | |
| Apoptosis assay | MEC1 | 0.01 uM | 24 hrs | Induction of apoptosis in human MEC1 cells assessed as decrease in MCL-1 level at 0.01 uM after 24 hrs by immunoblotting analysis | 30253346 | |
| Apoptosis assay | HL60 | 0.01 uM | 24 hrs | Induction of apoptosis in human HL60 cells assessed as decrease in MCL-1 level at 0.01 uM after 24 hrs by immunoblotting analysis | 30253346 | |
| Apoptosis assay | MV4-11 | 0.01 uM | 24 hrs | Induction of apoptosis in human MV4-11 cells assessed as decrease in c-MYC level at 0.01 uM after 24 hrs by immunoblotting analysis | 30253346 | |
| Apoptosis assay | MEC1 | 0.01 uM | 24 hrs | Induction of apoptosis in human MEC1 cells assessed as decrease in c-MYC level at 0.01 uM after 24 hrs by immunoblotting analysis | 30253346 | |
| Apoptosis assay | MV4-11 | 0.01 uM | 24 hrs | Induction of apoptosis in human MV4-11 cells assessed as decrease in MCL-1 level at 0.01 uM after 24 hrs by immunoblotting analysis | 30253346 | |
| Apoptosis assay | HL60 | 0.01 uM | 24 hrs | Induction of apoptosis in human HL60 cells assessed as decrease in c-MYC level at 0.01 uM after 24 hrs by immunoblotting analysis | 30253346 | |
| Cytotoxicity assay | U2OS | 96 hrs | IC50 = 0.006 μM | ChEMBL | ||
| Function assay | U2OS | 1 hr | IC50 = 0.007 μM | ChEMBL | ||
| Click to View More Cell Line Experimental Data | ||||||
Biological Activity
| Description | Dinaciclib is a novel and potent CDK inhibitor for CDK2, CDK5, CDK1 and CDK9 with IC50 of 1 nM, 1 nM, 3 nM and 4 nM in cell-free assays, respectively. It also blocks thymidine (dThd) DNA incorporation. Dinaciclib induces apoptosis through the activation of caspases 8 and 9. Phase 3. | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Targets |
|
| In vitro | ||||
| In vitro | Dinaciclib is also a potent DNA replication inhibitor that blocks thymidine (dThd) DNA incorporation in A2780 cells with IC50 of 4 nM. Dinaciclib strongly suppresses phosphorylation of Rb on Ser 807/811 at concentrations >6.25 nM, which is in agreement with the observation that 4 nM concentrations are required for 50% inhibition of dThd DNA incorporation in the same cell model. Significantly, complete suppression of Rb phosphorylation is correlated with the onset of apoptosis, as indicated by the appearance of the p85 PARP cleavage product in cells exposed to >6.25 nM Dinaciclib. Dinaciclib is active against a broad spectrum of human tumor cell lines. [1] Addition of Dinaciclib during exposure also suppresses accumulation of γ-H2AX, in a dose-dependent manner. [2] Dinaciclib inhibits melanoma cell proliferation, and drives melanoma cells into massive apoptosis. [3] Dinaciclib induces the apoptosis of several osteosarcoma cell lines including those resistant to doxorubicin. Dinaciclib attenuates the phosphorylation of RNAP II at serine 2 and the phosphorylation of the CDK inhibitor p27Kip1 at threonine 187. Reductions in phosphorylation activity occurrs at 12 - 40 nM Dinaciclib (4 to 16 hours post-Dinaciclib addition). Dinaciclib also reduces the phosphorylation of Rb at serine 807/811. Dinaciclib induces the apoptosis of mock- and p53-depleted U2OS cells to a similar extent. [4] |
|||
|---|---|---|---|---|
| Kinase Assay | Cyclin/CDK kinase assay | |||
| Recombinant cyclin/CDK holoenzymes are purified from Sf9 cells engineered to produce baculoviruses that express a specific cyclin or CDK. Cyclin/CDK complexes are typically diluted to a final concentration of 50 μg/mL in a kinase reaction buffer containing 50 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 1 mM DTT, and 0.1 mM sodium orthovanadate. For each kinase reaction, 1 μg of enzyme and 20 μL of a 2-μM substrate solution (a biotinylated peptide derived from histone H1) are mixed and combined with 10 μL of diluted Dinaciclib. The reaction is started by the addition of 50 μL of 2 μM ATP and 0.1 μCi of 33P-ATP. Kinase reactions are incubated for 1 hour at room temperature and are stopped by the addition of 0.1% Triton X-100, 1 mM ATP, 5 mM EDTA, and 5 mg/mL streptavidin-coated SPA beads. SPA beads are captured using a 96-well GF/B filter plate and a Filtermate universal harvester. Beads are washed twice with 2 M NaCl and twice with 2 M NaCl containing 1% phosphoric acid. The signal is then assayed using a TopCount 96-well liquid scintillation counter. | ||||
| Cell Research | Cell lines | A2780 cells | ||
| Concentrations | 0 μM -5 μM | |||
| Incubation Time | 24 hours | |||
| Method | A2780 cells are maintained in DMEM plus 10% fetal bovine serum and passaged twice weekly by detaching the monolayer with trypsin-EDTA. One hundred microliters of A2780 cells (5 × 103 cells) are added per well to a 96-well Cytostar-T plate and incubated for 16 hours to 24 hours at 37 °C. Dinaciclib is serially diluted in complete media plus 2% 14C-labeled dThd. Media are removed from the Cytostar T plate; 200 μL of various Dinaciclib dilutions are added in quadruplicate; and the cells are incubated for 24 hours at 37 °C. Accumulated incorporation of radiolabel is assayed using scintillation proximity and measured on a TopCounTM. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | Cleaved PARP / c-Myc Mcl-1 / Bcl-2 / Bcl-xl / Bax / Bak / PUMA / Noxa RNAP II (P-Ser2/P-Ser5) Survivin |
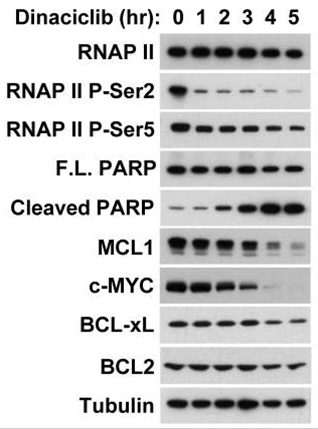
|
25289887 | |
| Growth inhibition assay | Cell viability Cell viability |
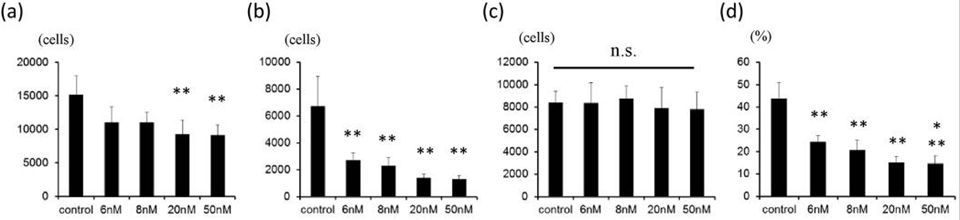
|
28361959 | |
| Immunofluorescence | cyclin B1 / α-tubulin / Aurora A OCT4 |
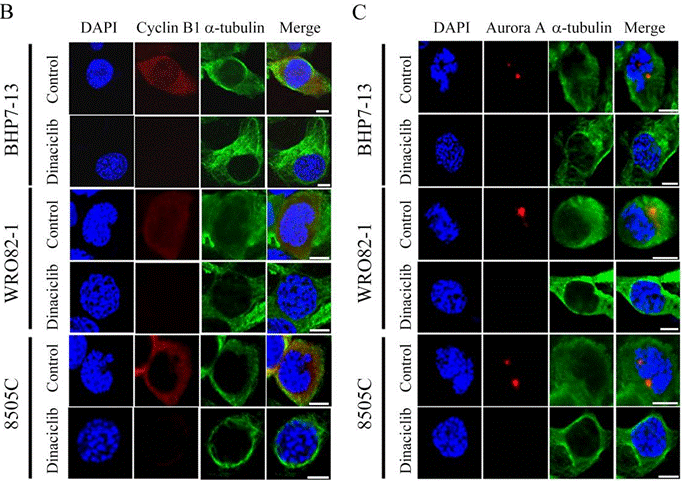
|
28207834 | |
| In Vivo | ||
| In vivo | Dinaciclib i.p. administration at 8, 16, 32, and 48 mg/kg daily for 10 days results in tumor inhibition by 70%, 70%, 89%, and 96%, respectively. Dinaciclib MED (minimum effective dose) appears to be <8 mg/kg. Dinaciclib is well tolerated, and the maximum body weight loss in the highest dosage group is 5%. Dinaciclib has dose-dependent antitumor activity in vivo, and that nearly complete inhibition of tumor growth occurs at a dose level below the MTD (maximum tolerated dose). Dinaciclib has a short plasma half-life in mouse. [1] |
|
|---|---|---|
| Animal Research | Animal Models | Nude mice bearing A2780 tumors |
| Dosages | 8 mg/kg, 16 mg/kg, 32 mg/kg, and 48 mg/kg | |
| Administration | Administered via i.p. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT03484520 | Terminated | Cancer - Acute Myeloid Leukemia |
AbbVie|Merck Sharp & Dohme LLC |
July 23 2018 | Phase 1 |
| NCT01434316 | Active not recruiting | Advanced Malignant Solid Neoplasm |
National Cancer Institute (NCI) |
November 1 2011 | Phase 1 |
|
Chemical Information & Solubility
| Molecular Weight | 396.49 | Formula | C21H28N6O2 |
| CAS No. | 779353-01-4 | SDF | Download Dinaciclib SDF |
| Smiles | CCC1=C2N=C(C=C(N2N=C1)NCC3=C[N+](=CC=C3)[O-])N4CCCCC4CCO | ||
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 79 mg/mL ( (199.24 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 35 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field
Frequently Asked Questions
Question 1:
I want to know how to reconstitute the inhibitor for in vivo studies?
Answer:
S2768 (SCH727965) in 15% Captisol at 8 mg/ml is a suspension for oral administration. And it can be dissolved in 2% DMSO/30% PEG 300/ddH2O at 10 mg/ml as a clear solution for injection.