Glycogen Synthase Kinase-3: Structure and Isoform-Specific Functions
Glycogen synthase kinase-3 (GSK-3) is a constitutively active kinase that was initially identified for its role in regulating glycogen synthesis by phosphorylating and inactivating glycogen synthase. Subsequent research has revealed that GSK-3 exists as two isoforms, GSK-3α (51 kDa) and GSK-3β (47 kDa), which are encoded by separate genes (GSK3A and GSK3B, respectively) and share approximately 97% homology in their catalytic domains but differ in their N- and C-terminal regions. These structural differences contribute to isoform-specific interactions with regulatory proteins and substrates, leading to distinct functional outcomes. GSK-3β, in particular, has been implicated in a wide range of disease-related pathways, making it a primary target for inhibitor development in research investigations.
Structural Basis of GSK-3 Kinase Activity
The catalytic domain of GSK-3 contains the conserved kinase fold, consisting of an N-terminal lobe rich in β-sheets and a C-terminal lobe dominated by α-helices, with the active site located at the interface between the two lobes. A unique feature of GSK-3 is its requirement for a "priming phosphate" on substrates, which binds to an arginine-rich pocket (the "priming site") adjacent to the active site, enabling efficient phosphorylation of the substrate at a serine or threonine residue four positions C-terminal to the priming phosphate. This priming-dependent mechanism distinguishes GSK-3 from many other kinases and dictates its substrate specificity. The constitutive activity of GSK-3 is attributed to the absence of an autoinhibitory domain, with regulation primarily occurring through post-translational modifications (e.g., phosphorylation, ubiquitination) and interactions with regulatory proteins.
Isoform-Specific Roles of GSK-3α and GSK-3β
While GSK-3α and GSK-3β share overlapping substrates and functions, accumulating evidence indicates isoform-specific roles in cellular processes and disease pathogenesis. GSK-3α is predominantly expressed in adipose tissue, liver, and brain, and has been linked to glycogen metabolism and insulin resistance. In contrast, GSK-3β is ubiquitously expressed and plays critical roles in inflammation, cell survival, and neurodegeneration. For example, in Alzheimer’s disease (AD), GSK-3β phosphorylates the microtubule-associated protein tau, leading to the formation of neurofibrillary tangles, a hallmark of AD pathology. In cancer, GSK-3β exhibits both tumor-promoting and tumor-suppressive roles depending on the cellular context, regulating the activity of oncogenes such as β-catenin and p53. These isoform-specific functions highlight the importance of developing selective GSK-3 inhibitors in research to dissect the distinct roles of GSK-3α and GSK-3β.
Pathway Modulation by GSK-3 Inhibitors: Key Signaling Networks
GSK-3 is a central node in numerous signaling pathways, integrating inputs from upstream regulators such as the PI3K/Akt, Wnt, and MAPK pathways. GSK-3 inhibitors exert their effects by disrupting these pathways, leading to downstream changes in gene expression and cellular function. Understanding the pathway-specific effects of GSK-3 inhibitors is critical for their application in research and therapeutic development, as it enables the identification of context-dependent outcomes and potential off-target effects.
Wnt/β-Catenin Pathway Regulation by GSK-3β Inhibitors
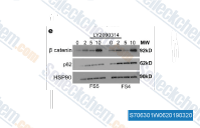
The Wnt/β-catenin pathway is one of the most well-characterized pathways regulated by GSK-3β. In the absence of Wnt signaling, GSK-3β forms a destruction complex with adenomatous polyposis coli (APC), axin, and casein kinase 1 (CK1), which phosphorylates β-catenin, targeting it for ubiquitination and proteasomal degradation. GSK-3β inhibitors disrupt this destruction complex, preventing β-catenin phosphorylation and leading to its accumulation in the cytoplasm and nucleus. Nuclear β-catenin then binds to T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors, activating the expression of target genes involved in cell proliferation and differentiation. This pathway modulation is particularly relevant in stem cell research, where GSK-3 inhibitors are used to maintain pluripotency, and in cancer research, where aberrant Wnt/β-catenin signaling drives tumorigenesis.
PI3K/Akt Pathway and GSK-3 Kinase Inhibition
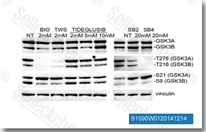
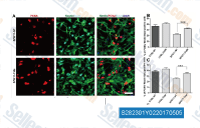
The PI3K/Akt pathway is a major upstream regulator of GSK-3, with Akt phosphorylating GSK-3α at Ser21 and GSK-3β at Ser9, leading to their inactivation. GSK-3 inhibitors mimic this inhibitory effect, bypassing upstream signaling events to directly block GSK-3 activity. This pathway modulation has significant implications in neurodegenerative disease research, as the PI3K/Akt/GSK-3 axis is dysregulated in AD, Parkinson’s disease, and Huntington’s disease. In preclinical studies, GSK-3 inhibitors have been shown to reduce tau phosphorylation, protect against neuronal apoptosis, and improve cognitive function in animal models of AD. Additionally, in metabolic research, GSK-3 inhibitors enhance insulin sensitivity by regulating glycogen synthesis and glucose uptake, making them potential candidates for the treatment of type 2 diabetes.
Substrates of GSK-3: Specificity and Regulatory Mechanisms in Inhibition
GSK-3 phosphorylates over 100 substrates, including transcription factors, cytoskeletal proteins, metabolic enzymes, and signaling molecules, highlighting its pleiotropic roles in cellular physiology. The specificity of GSK-3 inhibitors for substrate phosphorylation is a critical consideration in research, as off-target effects on non-GSK-3 substrates or isoform-specific substrates can complicate data interpretation. Understanding the regulatory mechanisms that govern GSK-3-substrate interactions is essential for the development of selective inhibitors and the accurate interpretation of their biological effects.
Substrate Specificity of GSK-3β: Priming-Dependent and Priming-Independent Mechanisms
As mentioned earlier, most GSK-3 substrates require a priming phosphate for efficient phosphorylation, a mechanism that contributes to substrate specificity. For example, glycogen synthase is primed by casein kinase 2, while β-catenin is primed by CK1. However, some substrates, such as p53 and heat shock protein 90 (Hsp90), are phosphorylated by GSK-3β in a priming-independent manner, expanding the range of cellular processes regulated by this kinase. GSK-3 inhibitors can block both priming-dependent and priming-independent phosphorylation, but the extent of inhibition varies depending on the inhibitor’s binding mode and specificity for GSK-3 isoforms. In research, this substrate specificity is exploited to dissect the role of individual GSK-3 substrates in disease pathways, for example, by using inhibitors to selectively block the phosphorylation of tau in neurodegeneration research.
Regulation of GSK-3 Substrate Phosphorylation by Inhibitors
The regulation of GSK-3 substrate phosphorylation by inhibitors is a complex process that involves not only direct inhibition of kinase activity but also indirect effects on upstream signaling pathways and regulatory proteins. For instance, some GSK-3 inhibitors bind to the active site of the kinase, competing with ATP and preventing substrate phosphorylation. Others bind to allosteric sites, inducing conformational changes that reduce kinase activity. Additionally, GSK-3 inhibitors can modulate the expression of regulatory proteins that interact with GSK-3, such as axin and APC, further influencing substrate phosphorylation. In research, techniques such as mass spectrometry and phospho-specific antibodies are used to characterize the substrate-specific effects of GSK-3 inhibitors, enabling the identification of novel downstream targets and the validation of inhibitor specificity.
GSK-3 Inhibitors in Scientific Research: Tools and Translational Potential
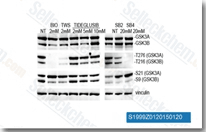
GSK-3 inhibitors have become indispensable tools in scientific research, facilitating the dissection of GSK-3-mediated pathways and the validation of GSK-3 as a therapeutic target. A wide range of GSK-3 inhibitors have been developed, including synthetic small molecules (e.g., SB216763, CHIR99021), natural products (e.g., lithium, curcumin), and peptide inhibitors. These inhibitors vary in their potency, selectivity for GSK-3 isoforms, and binding modes, making them suitable for different research applications. For example, CHIR99021, a selective GSK-3β inhibitor, is commonly used in stem cell research to maintain pluripotency, while SB216763 is used to study the role of GSK-3 in neurodegeneration.
Despite their utility in research, the translational potential of GSK-3 inhibitors has been hindered by challenges such as off-target effects, toxicity, and limited efficacy in clinical trials. However, recent advances in structure-based drug design have led to the development of more selective and potent GSK-3 inhibitors, addressing some of these limitations. For example, inhibitors that target the unique N-terminal region of GSK-3β have been shown to exhibit higher isoform specificity, reducing off-target effects on GSK-3α. Additionally, combination therapies involving GSK-3 inhibitors and other pathway modulators are being explored in cancer and neurodegenerative disease research, aiming to enhance efficacy and reduce toxicity.
In conclusion, GSK-3 inhibitors have significantly advanced our understanding of glycogen synthase kinase-3-mediated pathways, substrate regulation, and isoform-specific functions. As research continues to unravel the complex mechanisms underlying GSK-3 activity and the effects of inhibition, the development of more selective and effective GSK-3 inhibitors holds great promise for the treatment of a wide range of diseases. The ongoing integration of structural biology, proteomics, and preclinical models in GSK-3 inhibitor research will continue to drive scientific progress and translational success in this field.