- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
Ribociclib
CDK inhibitor
research use only
Ribociclib is an orally available, and highly specific inhibitor of CDK4 and CDK6 with IC50 of 10 nM and 39 nM. Phase 3.
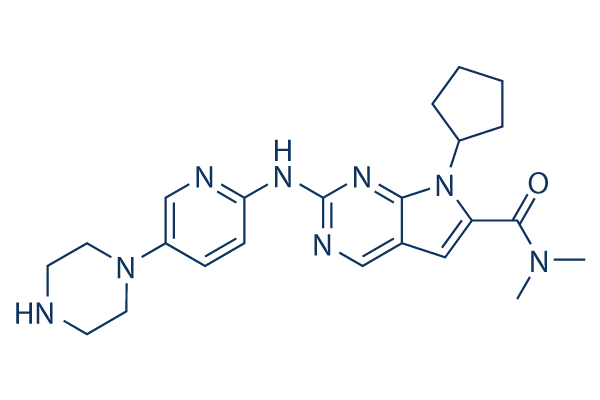
Ribociclib Chemical Structure
Molecular Weight: 434.54
Purity & Quality Control
Batch:
Purity:
99.99%
99.99
Ribociclib Related Products
| Related Targets | CDK1 CDK2 CDK3 CDK4 CDK5 CDK6 CDK7 CDK9 CLK Cdc CDK8 CDK12 CDK13 CDK19 CDK11 CDK/cyclin complexes | Click to Expand |
|---|---|---|
| Related Products | Ro-3306 Dinaciclib Roscovitine Flavopiridol (Alvocidib) SNS-032 (BMS-387032) Flavopiridol (Alvocidib) HCl AT7519 LDC000067 PHA-767491 HCl THZ1 2HCl XL413 JNJ-7706621 AZD5438 Purvalanol A Milciclib BS-181 HCl K03861 (AUZ454) PHA-793887 BMS-265246 AT7519 HCl | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library PI3K/Akt Inhibitor Library MAPK Inhibitor Library DNA Damage/DNA Repair compound Library Cell Cycle compound library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| DFSP105 | Growth Inhibition Assay | 24 h | GI50=276 nM | 25852058 | ||
| Myoblast | Growth Inhibition Assay | 72 h | IC50=1035 nM | 25810375 | ||
| IMRS | Growth Inhibition Assay | 72 h | IC50=873 nM | 25810375 | ||
| SKNAS | Growth Inhibition Assay | 72 h | IC50>10000 nM | 25810375 | ||
| Rh28 | Growth Inhibition Assay | 72 h | IC50=845 nM | 25810375 | ||
| Rh41 | Growth Inhibition Assay | 72 h | IC50=7187 nM | 25810375 | ||
| CW9019 | Growth Inhibition Assay | 72 h | IC50=9912 nM | 25810375 | ||
| Rh5 | Growth Inhibition Assay | 72 h | IC50>10000 nM | 25810375 | ||
| Rh30 | Growth Inhibition Assay | 72 h | IC50>10000 nM | 25810375 | ||
| 778 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| 449 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LP3 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LP6 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LP8 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| LPS141 | Growth Inhibition Assay | 72 h | inhibits cell growth dose dependently | 25028469 | ||
| 778 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| 449 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LP3 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LP6 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LP8 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| LPS141 | Growth Inhibition Assay | 3.33 μM | 24 h | decreases the proportion of cells in S phase | 25028469 | |
| IMR5 | Growth Inhibition Assay | 24 h | DMSO | IC50=126 nM | 24045179 | |
| BE2C | Growth Inhibition Assay | 24 h | DMSO | IC50=134 nM | 24045179 | |
| 1643 | Growth Inhibition Assay | 24 h | DMSO | IC50=147 nM | 24045179 | |
| SKNSH | Growth Inhibition Assay | 24 h | DMSO | IC50=148 nM | 24045179 | |
| SY5Y | Growth Inhibition Assay | 24 h | DMSO | IC50=154 nM | 24045179 | |
| NGP | Growth Inhibition Assay | 24 h | DMSO | IC50=175 nM | 24045179 | |
| KELLY | Growth Inhibition Assay | 24 h | DMSO | IC50=220 nM | 24045179 | |
| CHP134 | Growth Inhibition Assay | 24 h | DMSO | IC50=273 nM | 24045179 | |
| NLF | Growth Inhibition Assay | 24 h | DMSO | IC50=328 nM | 24045179 | |
| LAN5 | Growth Inhibition Assay | 24 h | DMSO | IC50=429 nM | 24045179 | |
| NB69 | Growth Inhibition Assay | 24 h | DMSO | IC50=738 nM | 24045179 | |
| SKNDZ | Growth Inhibition Assay | 24 h | DMSO | IC50=801 nM | 24045179 | |
| NBSD | Growth Inhibition Assay | 24 h | DMSO | IC50=1900 nM | 24045179 | |
| SKNF1 | Growth Inhibition Assay | 24 h | DMSO | IC50=3500 nM | 24045179 | |
| EBC1 | Growth Inhibition Assay | 24 h | DMSO | IC50=6400 nM | 24045179 | |
| SKNAS | Growth Inhibition Assay | 24 h | DMSO | IC50>10000 nM | 24045179 | |
| NB16 | Growth Inhibition Assay | 24 h | DMSO | IC50>10000 nM | 24045179 | |
| RPE1 | Growth Inhibition Assay | 24 h | DMSO | IC50>10000 nM | 24045179 | |
| Sf21 | Function assay | 10 mins | Inhibition of recombinant human full length N-terminal GST-tagged CDK4/Cyclin-D3 co-expressed in baculovirus infected sf21 cells using Rb substrate in presence of [gamma33P]ATP after 10 mins by scintillation counting method, IC50 = 0.013 μM. | 29518312 | ||
| Sf21 | Function assay | 10 mins | Inhibition of recombinant human full length C-terminal 6His-tagged CDK9/Cyclin-T1 co-expressed in baculovirus infected sf21 cells using PDKtide substrate in presence of [gamma33P]ATP after 10 mins by scintillation counting method, IC50 = 0.197 μM. | 29518312 | ||
| HepG2 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HepG2 cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.2862 μM. | 29407975 | ||
| SEM | Antiproliferative assay | 72 hrs | Antiproliferative activity against human SEM cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.4605 μM. | 29407975 | ||
| KOPN8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human KOPN8 cells after 72 hrs by CelTiter-Glo assay, EC50 = 0.5008 μM. | 29407975 | ||
| NCI-H1299 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human NCI-H1299 cells assessed as reduction in cell viability after 72 hrs by CCK8 assay, IC50 = 5.46 μM. | 29518312 | ||
| T47D | Growth inhibition assay | 72 hrs | Growth inhibition of human T47D cells incubated for 72 hrs by CCK8 assay, IC50 = 6.227 μM. | 28651979 | ||
| T47D | Cytotoxicity assay | 72 hrs | Cytotoxicity against human T47D cells assessed as reduction in cell viability after 72 hrs by CCK8 assay, IC50 = 6.23 μM. | 29518312 | ||
| H1299 | Growth inhibition assay | 72 hrs | Growth inhibition of human H1299 cells incubated for 72 hrs by CCK8 assay, IC50 = 7.637 μM. | 28651979 | ||
| KOPN8 | Apoptosis assay | 0.5 uM | 3 hrs | Induction of apoptosis in human KOPN8 cells assessed as upregulation of cleaved PARP level at 0.5 uM after 3 hrs by Western blot analysis | 29407975 | |
| KOPN8 | Apoptosis assay | 0.5 uM | 24 hrs | Induction of apoptosis in human KOPN8 cells assessed as upregulation of cleaved PARP level at 0.5 uM pre-treated with NAC for 1 hr and measured after 24 hrs by Western blot analysis | 29407975 | |
| Hep3B | Cell cycle assay | 24 hrs | Cell cycle arrest in human Hep3B cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| HepG2 | Cell cycle assay | 24 hrs | Cell cycle arrest in human HepG2 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| A549 | Cell cycle assay | 24 hrs | Cell cycle arrest in human A549 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| NCI-H460 | Cell cycle assay | 24 hrs | Cell cycle arrest in human NCI-H460 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| T47D | Cell cycle assay | 24 hrs | Cell cycle arrest in human T47D cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| MDA-MB-231 | Cell cycle assay | 24 hrs | Cell cycle arrest in human MDA-MB-231 cells assessed as accumulation at G0/G1 phase after 24 hrs by propidium iodide staining based flow cytometry | 29518312 | ||
| Fluc-labeled 4T1 | Antitumor assay | 130 mg/kg | 18 days | Antitumor activity against mouse Fluc-labeled 4T1 cells implanted in Balb/c mouse assessed as reduction in tumor weight at 130 mg/kg, ip administered daily for 18 days measured after 8 to 25 days | 29518312 | |
| T47D | Cell cycle assay | 24 hrs | Induction of cell cycle arrest in human T47D cells assessed as increase in G0/G1 phase accumulation incubated for 24 hrs by flow cytometry | 28651979 | ||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Description | Ribociclib is an orally available, and highly specific inhibitor of CDK4 and CDK6 with IC50 of 10 nM and 39 nM. Phase 3. | ||||
|---|---|---|---|---|---|
| Features | Orally bioavailable CDK4/6-selective inhibitor that has been tested in Phase III clinical trials for treatment of advanced breast cancer. | ||||
| Targets |
|
In vitro |
||||
| In vitro | LEE011, as dual CDK4/CDK6 inhibitor, significantly inhibits the growth of 12 of 17 neuroblastoma cell lines with mean IC50 of 307 nM. The growth inhibition of neuroblastoma cell lines is primarily cytostatic and is mediated by a G1 cell-cycle arrest and cellular senescence. [1] |
|||
|---|---|---|---|---|
| Cell Research | Cell lines | BE2C, IMR5, 1643, SY5Y, CHP134, SKNSH, NGP, KELLY, LAN5, NLF, NB69, SKNDZ, NBSD, NBLS, SKNFI, EBC1, SKNAS, NB16, RPE1 cell lines. | ||
| Concentrations | 10 μM | |||
| Incubation Time | ~100 hours | |||
| Method | A panel of neuroblastoma cell lines, selected based upon prior demonstration of substrate adherent growth, is plated in triplicate on the Xcelligence Real-Time Cell Electronic Sensing system and treated 24 hours later with a four-log dose range of inhibitor or with a dimethyl sulfoxide (DMSO) control. Cell indexes are monitored continuously for ~100 hours, and IC50 values are determined as follows: growth curves are generated by plotting the cell index as a function of time and are normalized to the cell index at the time of treatment for a baseline cell index of 1. The area under the normalized growth curve from the time of treatment to 96 hours posttreatment is then calculated using a baseline area of 1 (the cell index at the time of treatment). Areas are normalized to the DMSO control, and the resulting data are analyzed using a nonlinear log inhibitor versus normalized response function. All experiments are repeated at least once. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | pRb(S807) / Rb / p53 / Cyclin E / Cyclin D1 / CDK4 / CDK6 / p27 / p21 / PARP |
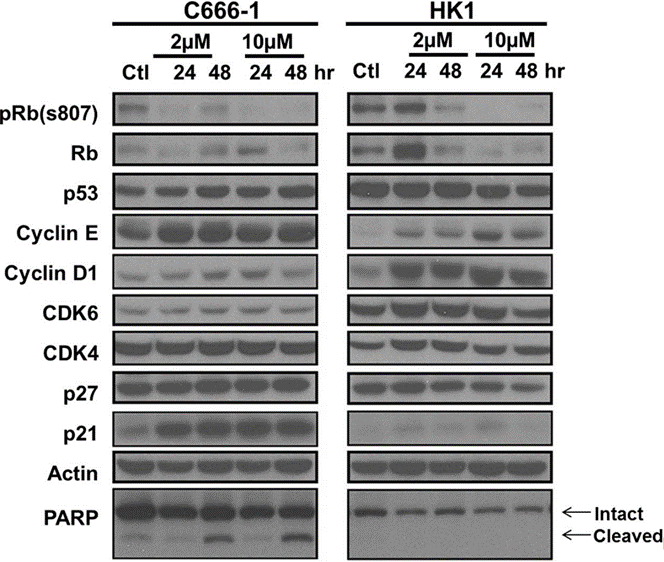
|
29789630 | |
| Growth inhibition assay | Cell viability |
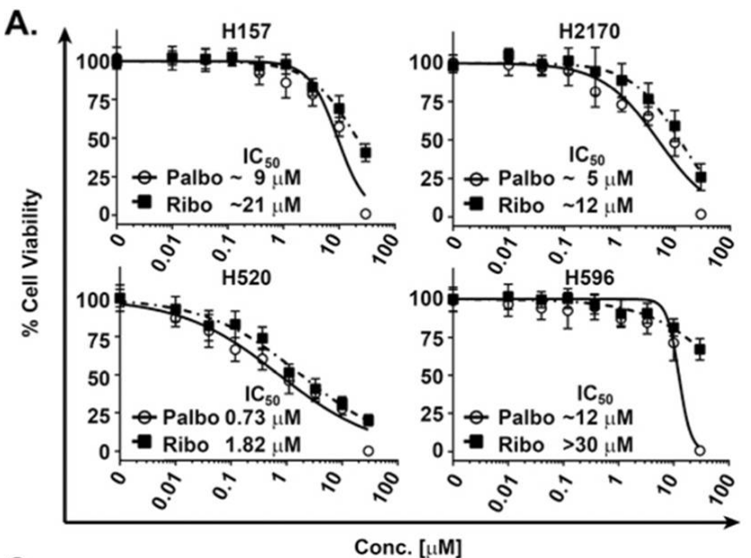
|
26390342 | |
In Vivo |
||
| In vivo | LEE011 (200 mg/kg daily, p.o.) significantly causes tumor growth delay in mice harboring the BE2C or 1643 xenografts with no weight loss or other signs of toxicity. [1] |
|
|---|---|---|
| Animal Research | Animal Models | Mice bearing BE2C, NB-1643, or EBC1 xenografts. |
| Dosages | ~200 mg/kg daily | |
| Administration | p.o. | |
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT05843253 | Not yet recruiting | High Grade Glioma|Diffuse Intrinsic Pontine Glioma|Anaplastic Astrocytoma|Glioblastoma|Glioblastoma Multiforme|Diffuse Midline Glioma H3 K27M-Mutant|Metastatic Brain Tumor|WHO Grade III Glioma|WHO Grade IV Glioma |
Nationwide Children''s Hospital|Novartis |
May 30 2024 | Phase 2 |
| NCT06075758 | Recruiting | Breast Cancer |
Novartis Pharmaceuticals|Novartis |
March 7 2024 | -- |
| NCT05996107 | Recruiting | Breast Cancer |
University of Michigan Rogel Cancer Center |
February 27 2024 | Phase 1 |
References |
|
Chemical Information
| Molecular Weight | 434.54 | Formula | C23H30N8O |
| CAS No. | 1211441-98-3 | SDF | Download Ribociclib SDF |
| Synonyms | LEE011 | ||
| Smiles | CN(C)C(=O)C1=CC2=CN=C(N=C2N1C3CCCC3)NC4=NC=C(C=C4)N5CCNCC5 | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 8 mg/mL ( (18.41 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Water : Insoluble Ethanol : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field