- Bioactive Compounds
- By Signaling Pathways
- PI3K/Akt/mTOR
- Epigenetics
- Methylation
- Immunology & Inflammation
- Protein Tyrosine Kinase
- Angiogenesis
- Apoptosis
- Autophagy
- ER stress & UPR
- JAK/STAT
- MAPK
- Cytoskeletal Signaling
- Cell Cycle
- TGF-beta/Smad
- DNA Damage/DNA Repair
- Compound Libraries
- Popular Compound Libraries
- Customize Library
- Clinical and FDA-approved Related
- Bioactive Compound Libraries
- Inhibitor Related
- Natural Product Related
- Metabolism Related
- Cell Death Related
- By Signaling Pathway
- By Disease
- Anti-infection and Antiviral Related
- Neuronal and Immunology Related
- Fragment and Covalent Related
- FDA-approved Drug Library
- FDA-approved & Passed Phase I Drug Library
- Preclinical/Clinical Compound Library
- Bioactive Compound Library-I
- Bioactive Compound Library-Ⅱ
- Kinase Inhibitor Library
- Express-Pick Library
- Natural Product Library
- Human Endogenous Metabolite Compound Library
- Alkaloid Compound LibraryNew
- Angiogenesis Related compound Library
- Anti-Aging Compound Library
- Anti-alzheimer Disease Compound Library
- Antibiotics compound Library
- Anti-cancer Compound Library
- Anti-cancer Compound Library-Ⅱ
- Anti-cancer Metabolism Compound Library
- Anti-Cardiovascular Disease Compound Library
- Anti-diabetic Compound Library
- Anti-infection Compound Library
- Antioxidant Compound Library
- Anti-parasitic Compound Library
- Antiviral Compound Library
- Apoptosis Compound Library
- Autophagy Compound Library
- Calcium Channel Blocker LibraryNew
- Cambridge Cancer Compound Library
- Carbohydrate Metabolism Compound LibraryNew
- Cell Cycle compound library
- CNS-Penetrant Compound Library
- Covalent Inhibitor Library
- Cytokine Inhibitor LibraryNew
- Cytoskeletal Signaling Pathway Compound Library
- DNA Damage/DNA Repair compound Library
- Drug-like Compound Library
- Endoplasmic Reticulum Stress Compound Library
- Epigenetics Compound Library
- Exosome Secretion Related Compound LibraryNew
- FDA-approved Anticancer Drug LibraryNew
- Ferroptosis Compound Library
- Flavonoid Compound Library
- Fragment Library
- Glutamine Metabolism Compound Library
- Glycolysis Compound Library
- GPCR Compound Library
- Gut Microbial Metabolite Library
- HIF-1 Signaling Pathway Compound Library
- Highly Selective Inhibitor Library
- Histone modification compound library
- HTS Library for Drug Discovery
- Human Hormone Related Compound LibraryNew
- Human Transcription Factor Compound LibraryNew
- Immunology/Inflammation Compound Library
- Inhibitor Library
- Ion Channel Ligand Library
- JAK/STAT compound library
- Lipid Metabolism Compound LibraryNew
- Macrocyclic Compound Library
- MAPK Inhibitor Library
- Medicine Food Homology Compound Library
- Metabolism Compound Library
- Methylation Compound Library
- Mouse Metabolite Compound LibraryNew
- Natural Organic Compound Library
- Neuronal Signaling Compound Library
- NF-κB Signaling Compound Library
- Nucleoside Analogue Library
- Obesity Compound Library
- Oxidative Stress Compound LibraryNew
- Plant Extract Library
- Phenotypic Screening Library
- PI3K/Akt Inhibitor Library
- Protease Inhibitor Library
- Protein-protein Interaction Inhibitor Library
- Pyroptosis Compound Library
- Small Molecule Immuno-Oncology Compound Library
- Mitochondria-Targeted Compound LibraryNew
- Stem Cell Differentiation Compound LibraryNew
- Stem Cell Signaling Compound Library
- Natural Phenol Compound LibraryNew
- Natural Terpenoid Compound LibraryNew
- TGF-beta/Smad compound library
- Traditional Chinese Medicine Library
- Tyrosine Kinase Inhibitor Library
- Ubiquitination Compound Library
-
Cherry Picking
You can personalize your library with chemicals from within Selleck's inventory. Build the right library for your research endeavors by choosing from compounds in all of our available libraries.
Please contact us at info@selleckchem.com to customize your library.
You could select:
- Antibodies
- Bioreagents
- qPCR
- 2x SYBR Green qPCR Master Mix
- 2x SYBR Green qPCR Master Mix(Low ROX)
- 2x SYBR Green qPCR Master Mix(High ROX)
- Protein Assay
- Protein A/G Magnetic Beads for IP
- Anti-Flag magnetic beads
- Anti-Flag Affinity Gel
- Anti-Myc magnetic beads
- Anti-HA magnetic beads
- Poly DYKDDDDK Tag Peptide lyophilized powder
- Protease Inhibitor Cocktail
- Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
- Phosphatase Inhibitor Cocktail (2 Tubes, 100X)
- Cell Biology
- Cell Counting Kit-8 (CCK-8)
- Animal Experiment
- Mouse Direct PCR Kit (For Genotyping)
- New Products
- Contact Us
SH-4-54
STAT inhibitor
research use only
SH-4-54 is a potent STAT inhibitor with KD of 300 nM and 464 nM for STAT3 and STAT5, respectively.
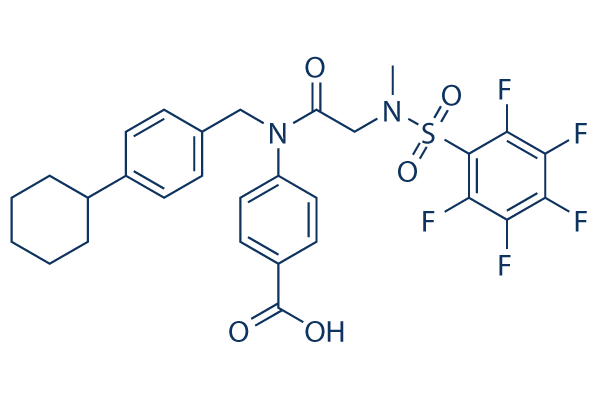
SH-4-54 Chemical Structure
Molecular Weight: 610.59
Purity & Quality Control
Batch:
Purity:
99.96%
99.96
SH-4-54 Related Products
| Related Targets | STAT1 STAT3 STAT5 STAT6 | Click to Expand |
|---|---|---|
| Related Products | Stattic NSC 74859 (S3I-201) Cryptotanshinone (Tanshinone C) C188-9 BP-1-102 Napabucasin (BBI608) HO-3867 Nifuroxazide AS1517499 Homoharringtonine (HHT) STAT5-IN-1 Ochromycinone (STA-21) HJC0152 APTSTAT3-9R STAT3-IN-1 SC-1 Narciclasine SH5-07 (SH-5-07) Scutellarin | Click to Expand |
| Related Compound Libraries | Kinase Inhibitor Library FDA-approved Drug Library Natural Product Library Tyrosine Kinase Inhibitor Library JAK/STAT compound library | Click to Expand |
Signaling Pathway
Cell Culture and Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| 127EF | Cytotoxicity assay | 3 days | Cytotoxicity against human 127EF cells assessed as cell viability after 3 days by Alamar Blue assay, IC50=0.066μM. | 24900612 | ||
| 30M | Cytotoxicity assay | 3 days | Cytotoxicity against human 30M cells assessed as cell viability after 3 days by Alamar Blue assay, IC50=0.1μM. | 24900612 | ||
| 84EF | Cytotoxicity assay | 3 days | Cytotoxicity against human 84EF cells assessed as cell viability after 3 days by Alamar Blue assay, IC50=0.102μM. | 24900612 | ||
| 67EF | Cytotoxicity assay | 3 days | Cytotoxicity against human 67EF cells assessed as cell viability after 3 days by Alamar Blue assay, IC50=0.106μM. | 24900612 | ||
| 73EF | Cytotoxicity assay | 3 days | Cytotoxicity against human 73EF cells assessed as cell viability after 3 days by Alamar Blue assay, IC50=0.162μM. | 24900612 | ||
| 25EF | Cytotoxicity assay | 3 days | Cytotoxicity against human 25EF cells assessed as cell viability after 3 days by Alamar Blue assay, IC50=0.234μM. | 24900612 | ||
| 30M | Cytotoxicity assay | 8 days | Cytotoxicity against human 30M cells assessed as cell viability after 8 days by Alamar Blue assay, IC50=0.43μM. | 24900612 | ||
| 73M | Cytotoxicity assay | 8 days | Cytotoxicity against human 73M cells assessed as cell viability after 8 days by Alamar Blue assay, IC50=1.03μM. | 24900612 | ||
| 147EF | Apoptosis assay | 0.1 to 1 uM | 3 hrs | Induction of apoptosis in human 147EF cells assessed as PARP cleavage at 0.1 to 1 uM after 3 hrs by Western blotting analysis | 24900612 | |
| 147EF | Function assay | 0.1 to 1 uM | 3 hrs | Inhibition of AKT phosphorylation in human 147EF cells at 0.1 to 1 uM after 3 hrs by Western blotting analysis | 24900612 | |
| 147EF | Function assay | 0.1 to 1 uM | 3 hrs | Inhibition of STAT3 phosphorylation at Y705 in human 147EF cells at 0.1 to 1 uM after 3 hrs by Western blotting analysis | 24900612 | |
| 73M | Function assay | 1 uM | Inhibition of STAT3 phosphorylation at Y705 in human 73M cells at 1 uM | 24900612 | ||
| 147EF | Function assay | 2 to 72 hrs | Inhibition of STAT3 in human 147EF cells assessed as reduction of phosphorylated Bcl-xL level after 2 to 72 hrs by Western blotting analysis | 24900612 | ||
| 147EF | Function assay | 2 to 72 hrs | Inhibition of STAT3 in human 147EF cells assessed as reduction of phosphorylated cyclin D1 level after 2 to 72 hrs by Western blotting analysis | 24900612 | ||
| BT73 | Function assay | 10 mg/kg | 3 days | In vivo inhibition of STAT3 phosphorylation in tumor of NOD-SCID mouse xenografted with human BT73 cells at 10 mg/kg, ip administered as 4 days on/3 days off schedule measured 2 hrs post last dose | 24900612 | |
| BT73 | Antitumor assay | 10 mg/kg | 3 days | Antitumor activity against human BT73 cells xenografted in NOD-SCID mouse assessed as decrease in tumor size at 10 mg/kg, ip administered as 4 days on/3 days off schedule measured 2 hrs post last dose by hematoxylin/eosin staining | 24900612 | |
| BT73 | Antitumor assay | 10 mg/kg | 3 days | Antitumor activity against human BT73 cells xenografted in NOD-SCID mouse assessed as induction of apoptosis in tumor at 10 mg/kg, ip administered as 4 days on/3 days off schedule measured 2 hrs post last dose by TUNEL assay | 24900612 | |
| BT73 | Antitumor assay | 10 mg/kg | 3 days | Antitumor activity against human BT73 cells xenografted in NOD-SCID mouse assessed as tumor growth inhibition by measuring downregulation of Ki67 protein expression at 10 mg/kg, ip administered as 4 days on/3 days off schedule measured 2 hrs post last dos | 24900612 | |
| Vero | Function assay | Vero cells viability counterscreen for qRT-PCR qHTS assay of selected Zika virus inhibitors | 33229545 | |||
| Click to View More Cell Line Experimental Data | ||||||
Mechanism of Action
| Description | SH-4-54 is a potent STAT inhibitor with KD of 300 nM and 464 nM for STAT3 and STAT5, respectively. | ||||
|---|---|---|---|---|---|
| Targets |
|
In vitro |
||||
| In vitro | SH-4-54 shows unprecedented cytotoxicity in human glioblastoma brain cancer stem cells (BTSCs), while has no toxicity in human fetal astrocytes. In addition, SH-4-54 effectively suppresses STAT3 phosphorylation and its downstream transcriptional targets. [1] | |||
|---|---|---|---|---|
| Kinase Assay | Surface Plasmon Resonance (SPR) studies | |||
| The binding experiments are carried out on a ProteOn XPR36 biosensor at 25°C using the HTE sensor chip. The flow cells of the sensor chip are loaded with a nickel solution at 30 μL/min for 120 s to saturate the Tris–NTA surface with Ni(II) ions. Purified His-tagged STAT3 and STAT5 in PBST buffer (PBS with 0.005% (v/v) Tween-20 and 0.001% DMSO pH 7.4) is injected in the first and second channels of the chip respectively in the vertical direction at a flow rate of 25 μg/μL for 300 s, which attained, on average, ~8000 resonance unit (RU). After a wash with PBST buffer, inhibitors binding to the immobilized proteins is monitored by injecting a range of concentrations along with a blank at a flow rate of 100 μL/min for 200 s for each of these small molecules. When the injection of the small molecule inhibitor is completed, running buffer is allowed to flow over the immobilized substrates for the non-specifically bound inhibitors to dissociate for 600 s. Following dissociation of the inhibitors, the chip surface is regenerated with an injection of 1 M NaCl at a flow rate of 100 μL/ml for 18 s. Interspot channel reference is used for non-specific binding corrections and the blank channel used with each analyte injection served as a double reference to correct for possible baseline drift. Data are analyzed using ProteOn Manager Software version 3.1. The Langmuir 1:1 binding model was used to determine the KD values. | ||||
| Cell Research | Cell lines | BTSC lines 25M, 67EF, 73EF, 84EF and 127EF | ||
| Concentrations | ~25 μM | |||
| Incubation Time | 72 hours | |||
| Method | BTSC spheres are dissociated to single cells with the enzyme Accumax, seeded at 1500 cells/ 96-well and treated with drug or vehicle (DMSO) one day after plating. Cytotoxicity studies are repeated independently using BTSC lines 25M, 67EF, 73EF, 84EF and 127EF. BTSC spheres are dissociated to single cells as above and plated in 96 well plates in triplicate at 3000 cells/ 96-well. In both sets of experiments drugs are used as serial dilutions within the range of 5 μM to 100 nM in the first set and 25 μM to 10 nM. Cell viability following drug treatment is assessed three days later using the alamarBlue assay according to the manufacturer’s instructions. All culture experiments are performed in triplicate with a minimum of three wells per condition. |
|||
| Experimental Result Images | Methods | Biomarkers | Images | PMID |
| Western blot | LMP1 / p-STAT3 / STAT3 |
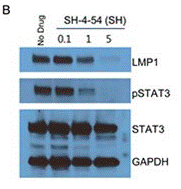
|
28445949 | |
In Vivo |
||
| In vivo | In mice orthotopically xenografted with BT73, SH-4-54 (10 mg/kg i.p.) exhibits BBB permeability, potently suppresses glioma tumor growth, and inhibits pSTAT3. [1] | |
|---|---|---|
| Animal Research | Animal Models | NOD-SCID bearing ed with BT73 glioma xenografts |
| Dosages | Suspended in 50% polyethylene glycol 300 in water | |
| Administration | i.p. | |
References |
|
Chemical Information
| Molecular Weight | 610.59 | Formula | C29H27F5N2O5S |
| CAS No. | 1456632-40-8 | SDF | Download SH-4-54 SDF |
| Synonyms | N/A | ||
| Smiles | CN(CC(=O)N(CC1=CC=C(C=C1)C2CCCCC2)C3=CC=C(C=C3)C(=O)O)S(=O)(=O)C4=C(C(=C(C(=C4F)F)F)F)F | ||
Storage and Stability
| Storage (From the date of receipt) | |||
|
In vitro |
DMSO : 100 mg/mL ( (163.77 mM) Moisture-absorbing DMSO reduces solubility. Please use fresh DMSO.) Ethanol : 100 mg/mL Water : Insoluble |
Molecular Weight Calculator |
|
In vivo Add solvents to the product individually and in order. |
In vivo Formulation Calculator |
|||||
Preparing Stock Solutions
Molarity Calculator
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
mg/kg
g
μL
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
% DMSO
%
% Tween 80
% ddH2O
%DMSO
%
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Tech Support
Answers to questions you may have can be found in the inhibitor handling instructions. Topics include how to prepare stock solutions, how to store inhibitors, and issues that need special attention for cell-based assays and animal experiments.
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
* Indicates a Required Field