research use only
Doxorubicin (Adriamycin) Hydrochloride DNA Topoisomerase II Inhibitor
Cat.No.S1208
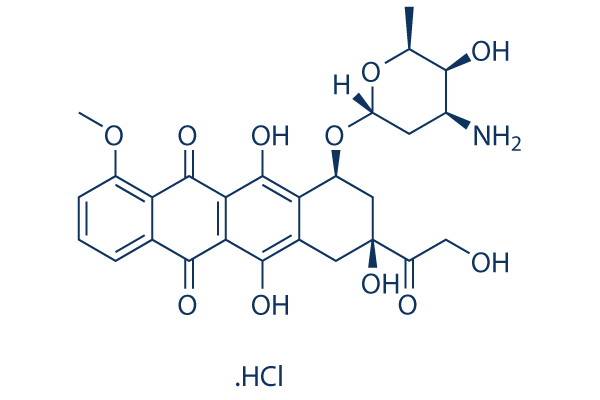
Chemical Structure
Molecular Weight: 579.98
Quality Control
| Related Targets | HDAC PARP ATM/ATR DNA-PK WRN DNA/RNA Synthesis Topoisomerase PPAR Sirtuin Casein Kinase |
|---|---|
| Other Antineoplastic and Immunosuppressive Antibiotics Inhibitors | Staurosporine (STS) Cyclosporin A Puromycin Dihydrochloride Oligomycin A (MCH 32) Geldanamycin (NSC 122750) Honokiol Streptozotocin (STZ) Cephalomannine Sodium Monensin (NSC 343257) 10-Deacetylbaccatin-III |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| SGC7901 | Growth Inhibition Assay | 10 μM | 72 h | DMSO | GI50=0.55 μM | 24686011 |
| HLF | Growth Inhibition Assay | 10 μM | 72 h | DMSO | GI50=0.33 μM | 24686011 |
| 16HBE | Growth Inhibition Assay | 10 μM | 72 h | DMSO | GI50=0.18 μM | 24650643 |
| BALB/3T3 | Growth Inhibition Assay | IC50=0.5 μM | 24631190 | |||
| 26-L5 | Function Assay | 6 h | Antiinvasive activity | 24592993 | ||
| CEM/ADR5000 | Cytotoxic Assay | DMSO | Cytotoxicity against human multidrug-resistant CEM/ADR5000 cells expressing p-glycoprotein with IC50 of 23.27 μM | 24561670 | ||
| CCRF-CEM | Cytotoxic Assay | DMSO | IC50=0.009 μM | 24561670 | ||
| V79 | Cytotoxic Assay | 1.10 μM | 72 h | DMSO | IC50=0.24 μM | 24530030 |
| MX1 | Cytotoxic Assay | 1.10 μM | 72 h | DMSO | IC50=0.5 μM | 24530030 |
| L929 | Cytotoxic Assay | 1.10 μM | 72 h | DMSO | IC50=0.16 μM | 24530030 |
| ME180 | Cytotoxic Assay | 48 h | IC50=0.39 μM | 24487188 | ||
| MGC803 | Cytotoxic Assay | 72 h | IC50=0.17 μM | 24370114 | ||
| CHP100 | Cytotoxic Assay | 72 h | IC50=0.79 μM | 24251417 | ||
| Bel7402 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.5782 μM | 24211639 | |
| SGC7901 | Cytotoxic Assay | IC50=0.011 μM | 24195466 | |||
| MKN28 | Growth Inhibition Assay | 48 h | GI50=0.24 μM | 24184076 | ||
| Caco2 | Growth Inhibition Assay | 48 h | GI50=0.32 μM | 24184076 | ||
| MDA-MB-468 | Growth Inhibition Assay | 10 μM | 72 h | GI50=0.085 μM | 24163729 | |
| Caco2 | Cytotoxic Assay | 40 μM | 24 h | Cytotoxicity | 24099994 | |
| U2OS | Growth Inhibition Assay | 72 h | IC50=0.3 μM | 24095089 | ||
| OVCAR8 | Growth Inhibition Assay | 20 μM | 72 h | Antiproliferative activity | 24095089 | |
| LNcaP | Function Assay | 3 μM | 24 h | Up-regulation of p53 expression | 24095089 | |
| LNcaP | Function Assay | 3 μM | 24 h | Increase of cleaved caspase-9 level | 24095089 | |
| Caov3 | Growth Inhibition Assay | 72 h | IC50=0.1 μM | 24095089 | ||
| AS49-RAW | Cytotoxic Assay | 100 μM | 48 h | IC50=32.78 μM | 24077528 | |
| MDA-MB-435 | Cytotoxic Assay | 100 μM | 48 h | IC50=0.15 μM | 24028446 | |
| HUVEC | Function Assay | 5 μM | 2 h | Cellular uptake | 24028446 | |
| MRC5 | Growth Inhibition Assay | 72 h | IC50=0.1 μM | 24021462 | ||
| HepG2 | Function Assay | 40 μg/mL | 48 h | DMSO | Inhibition of TRK with IC50 of 0.0025 μM | 24013411 |
| HUVEC | Cytotoxic Assay | 48 h | IC50=0.0807 μM | 24012378 | ||
| KB/HeLa | Cytotoxic Assay | 45 h | EC50=0.25 μM | 23988351 | ||
| MDA-MB-231 | Function Assay | 5 μM | 24 h | Antioxidant activity assessed as intramitochondrial ROS level | 23973213 | |
| MDA-MB-231 | Function Assay | 5 μM | 24 h | Antioxidant activity assessed as intracellular malonyldialdehyde level | 23973213 | |
| MDA-MB-231 | Function Assay | 5 μM | 24 h | Cellular uptake | 23973213 | |
| MCF7 | Function Assay | 5 μM | 24 h | Cellular uptake | 23973213 | |
| MCF7 | Function Assay | 5 μM | 24 h | Antioxidant activity assessed as intracellular malonyldialdehyde level | 23973213 | |
| MCF7 | Function Assay | 5 μM | 24 h | Cellular uptake | 23973213 | |
| MCF7 | Function Assay | 5 μM | 24 h | Antioxidant activity assessed as intramitochondrial ROS level | 23973213 | |
| H9c2 | Function Assay | 5 μM | 24 h | Cellular uptake | 23973213 | |
| H9c2 | Function Assay | 5 μM | 24 h | Antioxidant activity assessed as intracellular ROS level | 23973213 | |
| H9c2 | Function Assay | 5 μM | 24 h | Antioxidant activity assessed as intracellular malonyldialdehyde level | 23973213 | |
| H9c2 | Function Assay | 5 μM | 24 h | Antioxidant activity assessed as intramitochondrial ROS level | 23973213 | |
| HaCaT | Growth Inhibition Assay | 25 μg/mL | 48 h | TGI50=0.4 μM | 23876338 | |
| UO31 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.49 μM | 23871905 | |
| UACC257 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.14 μM | 23871905 | |
| U251 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.04 μM | 23871905 | |
| TK10 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.18 μM | 23871905 | |
| SR | Cytotoxic Assay | 100 μM | 48 h | GI50=0.03 μM | 23871905 | |
| SNB75 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.07 μM | 23871905 | |
| SNB19 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.04 μM | 23871905 | |
| SN12C | Cytotoxic Assay | 100 μM | 48 h | GI50=0.07 μM | 23871905 | |
| SK-MEL-28 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.21 μM | 23871905 | |
| SF539 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.12 μM | 23871905 | |
| RXF393 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.1 μM | 23871905 | |
| PRMI8226 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.08 μM | 23871905 | |
| OVCAR-8 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.1 μM | 23871905 | |
| OVCAR5 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.41 μM | 23871905 | |
| OVCAR4 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.37 μM | 23871905 | |
| NCI-H522 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.03 μM | 23871905 | |
| NCI-H322M | Cytotoxic Assay | 100 μM | 48 h | GI50=0.54 μM | 23871905 | |
| NCI-H226 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.05 μM | 23871905 | |
| MALME-3M | Cytotoxic Assay | 100 μM | 48 h | GI50=0.12 μM | 23871905 | |
| M14 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.18 μM | 23871905 | |
| LOXIMVI | Cytotoxic Assay | 100 μM | 48 h | GI50=0.07 μM | 23871905 | |
| KM12 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.27 μM | 23871905 | |
| IGROVI | Cytotoxic Assay | 100 μM | 48 h | GI50=0.17 μM | 23871905 | |
| HOP92 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.1 μM | 23871905 | |
| HCC2998 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.26 μM | 23871905 | |
| EKVX | Cytotoxic Assay | 100 μM | 48 h | GI50=0.41 μM | 23871905 | |
| Caki1 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.95 μM | 23871905 | |
| ACHN | Cytotoxic Assay | 100 μM | 48 h | GI50=0.08 μM | 23871905 | |
| 786-0 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.13 μM | 23871905 | |
| HOP62 | Growth Inhibition Assay | 100 μM | 48 h | GI50=0.14 μM | 23849207 | |
| Gurav | Growth Inhibition Assay | 100 μM | 48 h | GI50=0.17 μM | 23849207 | |
| DWD | Growth Inhibition Assay | 100 μM | 48 h | GI50=0.1 μM | 23849207 | |
| COLO205 | Growth Inhibition Assay | 100 μM | 48 h | GI50=0.14 μM | 23849207 | |
| LS180 | Cytotoxic Assay | 72 h | DMSO | IC50=0.04 μM | 23827179 | |
| 786 | Cytotoxic Assay | 48 h | DMSO | IC50=0.33 μM | 23810282 | |
| HGF | Cytotoxic Assay | 25 mM | 24 h | IC50=0.5 μM | 23802716 | |
| C643 | Cytotoxic Assay | 25 mM | 24 h | IC50=0.07 μM | 23802716 | |
| B16F10 | Cytotoxic Assay | 48 h | DMSO | IC50=0.072 μM | 23777898 | |
| JURKAT-TALL | Cytotoxic Assay | 48 h | IC50=0.0096 μM | 23673218 | ||
| U266 | Cytotoxic Assay | 72 h | IC50=0.67 μM | 23600925 | ||
| SW872 | Cytotoxic Assay | 72 h | IC50=0.14 μM | 23600925 | ||
| SK-UT-1 | Cytotoxic Assay | 72 h | IC50=0.076 μM | 23600925 | ||
| SK-ES-1 | Cytotoxic Assay | 72 h | IC50=0.042 μM | 23600925 | ||
| SK-BR-3 | Cytotoxic Assay | 72 h | IC50=0.09 μM | 23600925 | ||
| NCI-H929 | Cytotoxic Assay | 72 h | IC50=0.065 μM | 23600925 | ||
| MNNG-HOS | Cytotoxic Assay | 72 h | IC50=0.59 μM | 23600925 | ||
| MDA-MB-436 | Cytotoxic Assay | 72 h | IC50=0.1 μM | 23600925 | ||
| A673 | Apoptosis Assay | 24 h | DMSO | Induction of apoptosis assessed as caspase-3 activation with EC50 of 0.39 μM | 23600925 | |
| A673 | Cytotoxic Assay | 72 h | Cytotoxicity against human A673 cells after 72 hrs by resazurin/resorufin-based fluorescence assay with IC50 of 0.17 μM | 23600925 | ||
| MA9.3 | Cytotoxic Assay | 100 μM | 48 h | IC50=0.086 μM | 23578690 | |
| SK-MEL | Cytotoxic Assay | 48 h | DMSO | IC50=1.5 μM | 23547843 | |
| HeLa | Cytotoxic Assay | 1 μM | 72 h | IC50=0.01 μM | 23470139 | |
| POS1 | Growth Inhibition Assay | 72 h | IC50=0.043 μM | 23360521 | ||
| OSRGA | Growth Inhibition Assay | 72 h | IC50=0.093 μM | 23360521 | ||
| L929 | Growth Inhibition Assay | 72 h | IC50=0.161 μM | 23360521 | ||
| AT6-1 | Growth Inhibition Assay | 72 h | IC50=2 μM | 23360521 | ||
| WHCO1 | Cytotoxic Assay | 48 h | DMSO | IC50=0.5 μM | 23353747 | |
| MOLT4 | Cytotoxic Assay | 48 h | DMSO | IC50=0.02 μM | 23218718 | |
| MGC803 | Growth Inhibition Assay | 20 μM | 72 h | DMSO | IC50=0.7 μM | 23124210 |
| Bcap37 | Growth Inhibition Assay | 20 μM | 72 h | DMSO | IC50=1.28 μM | 23124210 |
| HL60/Vinc | Cytotoxic Assay | 100 μg/mL | 72 h | DMSO | Cytotoxicity against human HL60/Vinc cells with IC50 of 0.91 μM | 23079523 |
| BALB/3T3 | Cytotoxic Assay | 100 μg/mL | 72 h | DMSO | IC50=1.91 μM | 23079523 |
| MG63 | Cytotoxic Assay | IC50=2.7 μM | 23043498 | |||
| HL60/A | Cytotoxic Assay | 18 h | IC50=6.7 μM | 22985027 | ||
| A549 | Cytotoxic Assay | IC50=0.001 μM | 22951040 | |||
| MESSA/DX5 | Growth Inhibition Assay | 100 μg/mL | 72 h | DMSO | IC50=0.25 μM | 22944121 |
| SKLU1 | Cytotoxic Assay | 48 h | DMSO | IC50=0.0067 μM | 22858298 | |
| HCT15 | Cytotoxic Assay | 48 h | DMSO | IC50=0.005 μM | 22858298 | |
| U2OS | Cytotoxic Assay | 4 μM | 72 h | DMSO | IC50=3.4 μM | 22749279 |
| B16 | 48 h | IC50=0.03 μM | 22668451 | |||
| MKN45 | Cytotoxic Assay | 48 h | DMSO | IC50=0.168 μM | 22647723 | |
| HMEC1 | Cytotoxic Assay | 250 μg/mL | 72 h | IC50=0.06 μM | 22616554 | |
| BMDM | Cytotoxic Assay | 250 μg/mL | 72 h | IC50=4.6 μM | 22616554 | |
| J774A.1 | Cytotoxic Assay | 72 h | DMSO | IC50=0.02 μM | 22608675 | |
| WI38 | Growth Inhibition Assay | 100 μM | 72 h | DMSO | IC50=0.04 μM | 22555152 |
| SW620 | Growth Inhibition Assay | 100 μM | 72 h | DMSO | IC50=0.04 μM | 22555152 |
| NCI/ADR | Growth Inhibition Assay | 25 μg/mL | 48 h | IC50=13.5 μM | 22537680 | |
| 786-0 | Growth Inhibition Assay | 25 μg/mL | 48 h | IC50=2.1 μM | 22537680 | |
| SMMC7721 | Cytotoxic Assay | 500 μM | 48 h | DMSO | IC50=0.37 μM | 22537362 |
| KB/VCR | Cytotoxic Assay | 500 μM | 48 h | DMSO | IC50=0.45 μM | 22537362 |
| U87MG | Growth Inhibition Assay | 2 μM | 48 h | IC50=0.79 μM | 22515366 | |
| NCI-H290 | Growth Inhibition Assay | 2 μM | 48 h | IC50=0.32 μM | 22515366 | |
| LLC | Growth Inhibition Assay | 48 h | IC50=0.207 μM | 22515366 | ||
| XF498 | Cytotoxic Assay | 48 h | IC50=0.015 μM | 22483395 | ||
| SK-MEL-2 | Cytotoxic Assay | 48 h | IC50=0.002 μM | 22483395 | ||
| K562/A02 | Growth Inhibition Assay | 72 h | DMSO | Antiproliferative activity against multidrug-resistant human K562/A02 cells overexpressing p-glycoprotein with IC50 of 46.69 μM | 22450134 | |
| HuH7 | Growth Inhibition Assay | 25 ng/mL | 72 h | DMSO | Antiproliferative activity assessed as inhibition of BrdU incorporation | 22409771 |
| HuH7 | Function Assay | 25 ng/mL | 72 h | DMSO | Induction of senescence assessed as increase in senescence associated beta-galactosidase activity | 22409771 |
| SK-N-SH | Cytotoxic Assay | 48 h | IC50=0.97 μM | 22386528 | ||
| SiHa | Growth Inhibition Assay | GI50=0.14 μM | 22361684 | |||
| B16 | Cytotoxic Assay | 72 h | DMSO | IC50=0.1 μM | 22330634 | |
| SKOV3 | Function Assay | 5 μM | 1 h | DMSO | Cellular uptake in doxorubicin-resistant human SKOV3 cells | 22276998 |
| SKOV3 | Function Assay | 5 μM | 1 h | DMSO | Drug uptake in doxorubicin-resistant human SKOV3 cell nucleu | 22276998 |
| LNcaP | Apoptosis Assay | 20 μg/mL | 24 h | water | Induction of apoptosis assessed as increase in caspase 3/7 activation | 22264758 |
| PC3M | Cytotoxic Assay | 72 h | DMSO | IC50=0.24 μM | 22264149 | |
| QGY7701 | Cytotoxic Assay | 24 h | DMSO | IC50=8.65 μM | 22182926 | |
| A375-C5 | Growth Inhibition Assay | 48 h | DMSO | GI50=0.0915 μM | 22177409 | |
| RAW264.7 | Cytotoxic Assay | 10 μM | 24 h | DMSO | Cytotoxicity assessed as cell viability | 22129061 |
| SKOV3 | Growth Inhibition Assay | 50 μM | 72 h | DMSO | Antiproliferative activity against human SKOV3 cells overexpressing c-Src | 22119472 |
| BGC823 | Cytotoxic Assay | 96 h | DMSO | IC50=0.98 μM | 22070654 | |
| HCT8 | Cytotoxic Assay | 5 μg/mL | 72 h | DMSO | IC50=0.02 μM | 22000949 |
| MCF7 | Function Assay | 50 μg/mL | 48 h | DMSO | Reduction in AP-2 gamma mRNA expression | 22000946 |
| MCF7 | Function Assay | 50 μg/mL | 48 h | DMSO | Reduction in CYP19 mRNA expression | 22000946 |
| MCF7 | Function Assay | 50 μg/mL | 48 h | DMSO | Reduction in VEGF mRNA expression | 22000946 |
| Neuro2a | Cytotoxic Assay | 72 h | IC50=1.2 μM | 21996519 | ||
| PC3 | Growth Inhibition Assay | 48 h | GI50=0.09 μM | 21875046 | ||
| AGS | Growth Inhibition Assay | 48 h | GI50=0.01 μM | 21875046 | ||
| BT20 | Growth Inhibition Assay | 10 μM | 72 h | Antiproliferative activity | 21852023 | |
| K562/A02 | Cytotoxic Assay | 48 h | DMSO | IC50=17.84 μM | 21843940 | |
| Ca9-22 | Cytotoxic Assay | 72 h | DMSO | IC50=0.26 μM | 21800859 | |
| SH-SY5Y | Cytotoxic Assay | 48 h | IC50=0.5 μM | 21797280 | ||
| LAMA-84 | Cytotoxic Assay | 48 h | IC50=0.74 μM | 21797280 | ||
| A2780/ADR | Cytotoxic Assay | IC50=0.194 μM | 21774499 | |||
| A2780 | Cytotoxic Assay | IC50=0.0012 μM | 21774499 | |||
| NCI-H187 | Cytotoxic Assay | 72 h | IC50=0.03 μM | 21741833 | ||
| KATO | Cytotoxic Assay | 72 h | IC50=0.33 μM | 21741833 | ||
| ChaGo | Cytotoxic Assay | 72 h | IC50=0.83 μM | 21741833 | ||
| BT474 | Cytotoxic Assay | 72 h | IC50=0.07 μM | 21741833 | ||
| HFF | Cytotoxic Assay | 72 h | CC50=0.03 μM | 21741131 | ||
| Gurav | Cytotoxic Assay | 100 μM | 48 h | DMSO | GI50<0.1 μM | 21737288 |
| ZR-75B | Cytotoxic Assay | 72 h | IC50=2.02 μM | 21721528 | ||
| ZR-75B | Cytotoxic Assay | 72 h | IC50=0.04 μM | 21721528 | ||
| SW620 | Cytotoxic Assay | 72 h | IC50=0.01 μM | 21721528 | ||
| NIH3T3 | Cytotoxic Assay | 72 h | IC50=0.05 μM | 21721528 | ||
| NCI/ADR-RES | Cytotoxic Assay | 72 h | IC50=9.3 μM | 21721528 | ||
| LLC-PK1 | Cytotoxic Assay | 72 h | IC50=0.2 μM | 21721528 | ||
| KBV1 | Cytotoxic Assay | 72 h | IC50=4.7 μM | 21721528 | ||
| KB3-1 | Cytotoxic Assay | 72 h | IC50=0.02 μM | 21721528 | ||
| HEK293 | Cytotoxic Assay | 72 h | IC50=0.42 μM | 21721528 | ||
| HEK293 | Cytotoxic Assay | 72 h | IC50=0.01 μM | 21721528 | ||
| Ew36 | Cytotoxic Assay | 72 h | IC50=0.01 μM | 21721528 | ||
| MG63 | Growth Inhibition Assay | 72 h | IC50=2.8 μM | 21721519 | ||
| PC3 | Cytotoxic Assay | 160 μg/mL | 5 d | DMSO | GI50=0.021 μM | 21711054 |
| NCI-H23 | Cytotoxic Assay | 160 μg/mL | 5 d | DMSO | GI50=0.005 μM | 21711054 |
| Hs578 | Cytotoxic Assay | 160 μg/mL | 5 d | DMSO | GI50=0.006 μM | 21711054 |
| BT549 | Cytotoxic Assay | 160 μg/mL | 5 d | DMSO | GI50=0.01 μM | 21711054 |
| HOP62 | Cytotoxic Assay | 24 h | GI50=0.14 μM | 21676506 | ||
| SW480 | Growth Inhibition Assay | 48 h | IC50=0.06 μM | 21641694 | ||
| S180 | Growth Inhibition Assay | 48 h | IC50=29.2 μM | 21620529 | ||
| H22 | Growth Inhibition Assay | 48 h | IC50=36.51 μM | 21620529 | ||
| P388D1 | Growth Inhibition Assay | IC50=0.338 μM | 21570750 | |||
| KATOIII | Growth Inhibition Assay | IC50=4.38 μM | 21570750 | |||
| SK-MEL-28 | Growth Inhibition Assay | 25 nM | 24 h | DMSO | IC50=0.6 μM | 21553829 |
| SF295 | Growth Inhibition Assay | 25 nM | 24 h | DMSO | IC50=4.4 μM | 21553829 |
| A375 | Growth Inhibition Assay | 72 h | PBS | IC50=0.13 μM | 21496972 | |
| HCT8 | Cytotoxic Assay | 0.58 μg/mL | 69 h | DMSO | IC50=0.02 μM | 21381705 |
| NCI-N87 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.4198 μM | 21296467 | |
| HUVEC | Growth Inhibition Assay | 96 h | DMSO | IC50=0.0303 μM | 21296467 | |
| HepG2 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.019 μM | 21296467 | |
| CCRF | Growth Inhibition Assay | 96 h | DMSO | IC50=0.0375 μM | 21296467 | |
| IMR90 | Cytotoxic Assay | 48 h | DMSO | IC50=0.59 μM | 21215623 | |
| Molt4/C8 | Cytotoxic Assay | 72 h | DMSO | IC50=0.2 μM | 21144624 | |
| CEM | Cytotoxic Assay | 72 h | DMSO | IC50=0.06 μM | 21144624 | |
| MDA435 | Cytotoxic Assay | 10 μM | 72 h | DMSO | IC50=0.0035 μM | 21142180 |
| MDA231 | Cytotoxic Assay | 10 μM | 72 h | DMSO | IC50=4.7x10-5 μM | 21142180 |
| HL60R | Cytotoxic Assay | 10 μM | 72 h | DMSO | IC50=0.99 μM | 21142180 |
| C33A | Cytotoxic Assay | 100 μg/mL | 48 h | IC50=0.82 μM | 21071221 | |
| MKN45 | Growth Inhibition Assay | 72 h | IC50=0.00019 μM | 21067930 | ||
| KB | Growth Inhibition Assay | 72 h | IC50=0.00023 μM | 21067930 | ||
| H460 | Growth Inhibition Assay | 72 h | IC50=0.00038 μM | 21067930 | ||
| KB8.5 | Cytotoxic Assay | 50 μM | 72 h | DMSO | IC50=3.3 μM | 21035234 |
| BJ | Cytotoxic Assay | 48 h | DMSO | IC50=2.33 μM | 20931970 | |
| ARPE19 | Cytotoxic Assay | 48 h | DMSO | IC50=0.76 μM | 20931970 | |
| THP1 | Cytotoxic Assay | 72 h | DMSO | IC50=0.008 μM | 20833057 | |
| SiHa | Cytotoxic Assay | 4 μM | 24 h | GI50=0.17 μM | 20732817 | |
| DWD | Cytotoxic Assay | 4 μM | 24 h | GI50=0.1 μM | 20732817 | |
| SKOV3 | Cytotoxic Assay | IC50=0.001 μM | 20719504 | |||
| Hep3B | Cytotoxic Assay | 11 d | IC50=0.14 μM | 20718475 | ||
| COLO205 | Cytotoxic Assay | 48 h | DMSO | GI50=0.1 μM | 20673627 | |
| HCT-15 | Cytotoxic Assay | IC50=0.038 μM | 20594848 | |||
| NUGC3 | Cytotoxic Assay | GI50=0.202 μM | 20579876 | |||
| CCRF-CEM | Growth Inhibition Assay | 48 h | GI50=0.02 μM | 20570145 | ||
| HAEC | Cytotoxic Assay | 100 μM | 24 h | DMSO | IC50=13 μM | 20547797 |
| PAXF1657L | Cytotoxic Assay | 10 μM | 24 h | IC50=0.052 μM | 20545334 | |
| MEXF462NL | Cytotoxic Assay | 10 μM | 24 h | IC50=0.052 μM | 20545334 | |
| MAXF401NL | Cytotoxic Assay | 10 μM | 24 h | IC50=0.052 μM | 20545334 | |
| GXF251L | Cytotoxic Assay | 10 μM | 24 h | IC50=0.052 μM | 20545334 | |
| CEM/ADR5000 | Function Assay | 1 μg/mL | 6 h | Increase in ROS generation | 20527917 | |
| CEM/ADR5000 | Apoptosis Assay | 1 μg/mL | 24 h | Induction of apoptosis | 20527917 | |
| PCO3 | Cytotoxic Assay | 250 μg/mL | 48 h | GI50=0.17 μM | 20413188 | |
| OVCAR-3 | Cytotoxic Assay | 250 μg/mL | 48 h | GI50=0.07 μM | 20413188 | |
| NCI/ADR | Cytotoxic Assay | 250 μg/mL | 48 h | GI50=5.99 μM | 20413188 | |
| 3T3 | Cytotoxic Assay | 10 μM | 48 h | IC50=3.7 μM | 20405844 | |
| HBI10A | Cytotoxic Assay | CC50<0.3 μM | 20363140 | |||
| U937 | Cytotoxic Assay | 72 h | IC50=0.00445 μM | 20356655 | ||
| NB-1 | Cytotoxic Assay | 72 h | IC50=0.00515 μM | 20356655 | ||
| K562 | Cytotoxic Assay | 72 h | IC50=0.00612 μM | 20356655 | ||
| HL60 | Cytotoxic Assay | 72 h | IC50=0.00113 μM | 20356655 | ||
| GOTO | Cytotoxic Assay | 72 h | IC50=0.00473 μM | 20356655 | ||
| SK-N-MC | Cytotoxic Assay | 72 h | IC50=0.12 μM | 20303768 | ||
| U87MG | Cytotoxic Assay | 48 h | IC50=0.018 μM | 20210346 | ||
| SKHEP1 | Cytotoxic Assay | 48 h | IC50=0.01 μM | 20210346 | ||
| MIAPaCa2 | Cytotoxic Assay | 100 μM | 72 h | IC50=0.01 μM | 20207049 | |
| SKHep | Growth Inhibition Assay | 48 h | DMSO | IC50=0.09 μM | 20171108 | |
| MESSA/DX5 | Function Assay | 50 μM | 2 h | water | Induction of DNA damage assessed as increase in comet-tail length | 20151639 |
| MESSA | Function Assay | 25 μM | 2 h | water | Cellular uptake | 20151639 |
| MESSA | Function Assay | 50 μM | 2 h | water | Induction of DNA damage assessed as increase in comet-tail length | 20151639 |
| MCF10A | Cytotoxic Assay | 40 μM | 48 h | DMSO | IC50=0.18 μM | 20064676 |
| IMR32 | Growth Inhibition Assay | 48 h | DMSO | IC50=1.7 μM | 19939522 | |
| MOLT3 | Cytotoxic Assay | IC50=0.04 μM | 19824618 | |||
| HuCCa1 | Cytotoxic Assay | 4 d | IC50=0.64 μM | 19824618 | ||
| HCT15 | Cytotoxic Assay | IC50=0.037 μM | 19819696 | |||
| MDA-MB-453 | Cytotoxic Assay | 0.58 μg/mL | 69 h | DMSO | IC50=0.12 μM | 19788290 |
| HL60/MX2 | Cytotoxic Assay | 48 h | DMSO | IC50=8.046 μM | 19743858 | |
| 2008/P | Cytotoxic Assay | 5 d | DMSO | IC50=0.063 μM | 19725578 | |
| 2008/MRP1 | Cytotoxic Assay | 5 d | DMSO | IC50=0.577 μM | 19725578 | |
| Colon 26-L5 | Cytotoxic Assay | 72 h | DMSO | IC50=3.12 μM | 19689125 | |
| B16-BL6 | Cytotoxic Assay | 72 h | DMSO | IC50=3.07 μM | 19689125 | |
| KB | Cytotoxic Assay | 72 h | DMSO | IC50=0.001 μM | 19655762 | |
| HCT116 | Cytotoxic Assay | 72 h | DMSO | IC50=0.006 μM | 19655762 | |
| U251 | Growth Inhibition Assay | 72 h | DMSO | IC50=0.6 μM | 19632833 | |
| OS-RC2 | Growth Inhibition Assay | 72 h | DMSO | IC50=5.4 μM | 19632833 | |
| MOLT4 | Growth Inhibition Assay | 72 h | DMSO | IC50=0.002 μM | 19632833 | |
| L78 | Growth Inhibition Assay | 72 h | DMSO | IC50=2.4 μM | 19632833 | |
| IMR90 | Growth Inhibition Assay | 72 h | DMSO | IC50=1 μM | 19632833 | |
| SK-N-SH | Apoptosis Assay | 5 μM | Induction of apoptosis assessed as caspase 3 activity | 19631549 | ||
| SK-N-SH | Apoptosis Assay | 5 μM | Induction of apoptosis at 0.5 to 5 uM | 19631549 | ||
| SNU1 | Cytotoxic Assay | IC50=4 μM | 19585998 | |||
| MDA-MB-231 | Growth Inhibition Assay | 200 μM | 48 h | DMSO | Antitumor activity against human estrogen receptor deficient MDA-MB-231 cells with GI50 of 0.01086 μM | 19428155 |
| U251 | Function Assay | 1 μM | 16 h | DMSO | Inhibition of HIF1 activation in human U251 cells stably transfected in pGL2-TK-HRE plasmid with EC50 of 0.12 μM | 19405508 |
| SKOV3 | Growth Inhibition Assay | GI50=0.021 μM | 19394218 | |||
| MESSA | Growth Inhibition Assay | GI50=0.0051 μM | 19394218 | |||
| A549 | Growth Inhibition Assay | GI50=0.003 μM | 19394218 | |||
| A431 | Growth Inhibition Assay | GI50=0.0012 μM | 19394218 | |||
| PA1 | Cytotoxic Assay | 10 μM | 72 h | IC50=0.63 μM | 19361894 | |
| HBL100 | Cytotoxic Assay | 10 μM | 72 h | IC50=0.24 μM | 19361894 | |
| ECV304 | Cytotoxic Assay | 10 μM | 72 h | Cytotoxicity | 19361894 | |
| WTK1 | Function Assay | 0.1 μM | 72 h | Cell cycle arrest in human WTK1 cells expressing p53 M237I mutant assessed as accumulation at sub-G0/G1 phase | 19349174 | |
| WTK1 | Function Assay | 25 μM | 8 h | Induction of p53 transcriptional activity in human WTK1 cells expressing p53 M237I mutant assessed as p21-Cip1/Waf1 expression | 19349174 | |
| WTK1 | Function Assay | 25 μM | 8 h | Induction of p53 phosphorylation at Ser15 in human WTK1 cells expressing p53 M237I mutant | 19349174 | |
| WTK1 | Function Assay | 0.1 μM | 8 h | Induction of p53 transcriptional activity in human WTK1 cells expressing p53 M237I mutant assessed as p21-Cip1/Waf1 expression | 19349174 | |
| WTK1 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in human WTK1 cells expressing p53 M237I mutant assessed as increase in PARP cleavage | 19349174 | |
| WTK1 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in human WTK1 cells expressing p53 M237I mutant assessed as decrease in pro-caspase 3 level | 19349174 | |
| WTK1 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in human WTK1 cells expressing p53 M237I mutant assessed as increase in cleaved caspase 3 level | 19349174 | |
| WTK1 | Function Assay | 0.1 μM | 8 h | Induction of p53 phosphorylation at Ser15 in human WTK1 cells expressing p53 M237I mutant | 19349174 | |
| TK6 | Function Assay | 25 μM | 8 h | Induction of p53 transcriptional activity in human TK6 cells expressing wild type p53 assessed as p21-Cip1/Waf1 expression | 19349174 | |
| TK6 | Function Assay | 25 μM | 8 h | Induction of p53 phosphorylation at Ser15 in human TK6 cells expressing wild type p53 | 19349174 | |
| TK6 | Function Assay | 0.1 μM | 72 h | Cell cycle arrest in human TK6 cells expressing wild type p53 assessed as accumulation at sub-G0/G1 phase | 19349174 | |
| TK6 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in human TK6 cells expressing wild type p53 assessed as increase in PARP cleavage | 19349174 | |
| TK6 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in human TK6 cells expressing wild type p53 assessed as decrease in pro-caspase 3 level | 19349174 | |
| TK6 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in human TK6 cells expressing wild type p53 assessed as increase in cleaved caspase 3 level | 19349174 | |
| TK6 | Function Assay | 0.1 μM | 8 h | Induction of p53 transcriptional activity in human TK6 cells expressing wild type p53 assessed as p21-Cip1/Waf1 expression | 19349174 | |
| TK6 | Function Assay | 0.1 μM | 8 h | Induction of p53 phosphorylation at Ser15 in human TK6 cells expressing wild type p53 | 19349174 | |
| NH32 | Growth Inhibition Assay | 0.1 μM | 72 h | Cell cycle arrest in p53 deficient human NH32 cells assessed as accumulation at sub-G0/G1 phase | 19349174 | |
| NH32 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in p53 deficient human NH32 cells assessed as increase in PARP cleavage | 19349174 | |
| NH32 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in p53 deficient human NH32 cells assessed as decrease in pro-caspase 3 level | 19349174 | |
| NH32 | Apoptosis Assay | 0.1 μM | 8 h | Induction of apoptosis in p53 deficient human NH32 cells assessed as increase in cleaved caspase 3 level | 19349174 | |
| SMMC-7541 | Cytotoxic Assay | 72 h | IC50=0.0037 μM | 19285863 | ||
| T47D | Apoptosis Assay | 10 μM | 48 h | DMSO | Induction of apoptosis assessed as caspase activation with EC50 of 0.57 μM | 19282188 |
| SNU398 | Apoptosis Assay | 10 μM | 48 h | DMSO | Induction of apoptosis assessed as caspase activation with EC50 of 0.39 μM | 19282188 |
| MEF | Cytotoxic Assay | 72 h | IC50=3.4 μM | 19186946 | ||
| Hs888Lu | Cytotoxic Assay | 72 h | IC50=1.4 μM | 19186946 | ||
| HL60 | Cytotoxic Assay | 100 μM | 72 h | DMSO | IC50=0.018 μM | 19136263 |
| A2780/DX3 | Function Assay | 10 μM | 2 h | saline | Reversal of P-gp 170-mediated multidrug resistance assessed as increase in intracellular doxorubicin accumulation | 19093883 |
| XF498 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.16 μM | 19053767 | |
| MT4 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.003 μM | 19053767 | |
| HT-29 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.002 μM | 19053767 | |
| HT-29 | Cytotoxic Assay | 72 h | DMSO | IC50=0.018 μM | 19028425 | |
| Bel7402 | Cytotoxic Assay | 72 h | DMSO | IC50=0.021 μM | 19028425 | |
| DU145 | Cytotoxic Assay | 25 μg/mL | 72 h | IC50=0.0039 μM | 18829316 | |
| MRC5 | Cytotoxic Assay | 72 h | IC50=0.1 μM | 18783950 | ||
| Jurkat | Cytotoxic Assay | 72 h | IC50=0.03 μM | 18783950 | ||
| AW8507 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.17 μM | 18657979 | |
| AW13516 | Cytotoxic Assay | 100 μM | 48 h | GI50=0.1 μM | 18657979 | |
| SK-MEL-2 | Growth Inhibition Assay | 72 h | GI50=0.11 μM | 18585035 | ||
| L1210 | Function Assay | 0.5 μg/mL | 12 h | water | Cell cycle arrest in mouse L1210 cells by accumulation at G2/M phase | 18508273 |
| LLC | Cytotoxic Assay | 72 h | DMSO | IC50=0.01 μM | 18440233 | |
| Colon 26-L5 | Cytotoxic Assay | 72 h | DMSO | IC50=0.01 μM | 18440233 | |
| MCF7/DX | Cytotoxic Assay | 24 h | water | Cytotoxicity against human doxorubicin-resistant MCF7/Dx cells with IC50 of 5.3 μM | 18429610 | |
| Caco2 | Function Assay | 50 nM | 48 h | water | Decrease in cyclin A expression | 18429610 |
| SNU638 | Cytotoxic Assay | 72 h | DMSO | IC50=0.052 μM | 18321715 | |
| HT1080 | Cytotoxic Assay | 72 h | DMSO | IC50=0.022 μM | 18321715 | |
| Hep2 | Cytotoxic Assay | 24 h | DMSO | IC50=0.42 μM | 18281125 | |
| A375 | Cytotoxic Assay | 24 h | DMSO | IC50=0.01 μM | 18281125 | |
| K/VP.5 | Growth Inhibition Assay | 72 h | DMSO | Growth inhibition of human K/VP.5 cells with IC50 of 0.41 μM | 18258442 | |
| PC3M | Growth Inhibition Assay | 72 h | DMSO | IC50=1.11 μM | 18247573 | |
| SJSA1 | Cytotoxic Assay | 1 μM | 6 h | Cytotoxicity against human SJSA1 cells expressing GRP78 with IC50 of 0.0062 μM | 18243696 | |
| HepG2 | Cytotoxic Assay | 100 μM | 72 h | IC50=0.07 μM | 18181575 | |
| U373MG | Growth Inhibition Assay | 24 h | DMSO | Growth inhibition of human U373MG cells containing UhiCE expressing plasmid with IC50 of 0.19953 μM | 18173233 | |
| U373MG | Growth Inhibition Assay | 24 h | DMSO | Growth inhibition of human U373MG cells containing UhCE1 expressing plasmid with IC50 of 0.15849 μM | 18173233 | |
| U373MG | Growth Inhibition Assay | 24 h | DMSO | Growth inhibition of human U373MG cells containing U373IRES plasmid with IC50 of 0.15849 μM | 18173233 | |
| SNU638 | Growth Inhibition Assay | 72 h | IC50=0.07 μM | 18063366 | ||
| SF268 | Growth Inhibition Assay | 72 h | DMSO | IC50=0.04 μM | 18052326 | |
| W301 | Growth Inhibition Assay | 50 mM | 48 h | DMSO | Growth inhibition of Saccharomyces cerevisiae W301 with IC50 of 10 μM | 18040043 |
| Daudi | Cytotoxic Assay | 100 μM | 96 h | Cytotoxicity against human Daudi cells under deem light with IC50 of 0.3 μM | 17993275 | |
| 2780.9S | Cytotoxic Assay | 100 μM | 96 h | Cytotoxicity against human 2780.9S cells under deem light with IC50 of 15 μM | 17993275 | |
| MCF7 | Growth Inhibition Assay | 100 μM | 72 h | DMSO | IC50=0.0007 μM | 17949859 |
| KB3.1 | Growth Inhibition Assay | 100 μM | 72 h | DMSO | IC50=0.0004 μM | 17949859 |
| WI38 | Growth Inhibition Assay | 100 μM | 72 h | IC50=0.1 μM | 17935309 | |
| MIAPaCa2 | Growth Inhibition Assay | 100 μM | 72 h | IC50=0.024 μM | 17935309 | |
| UACC257 | Growth Inhibition Assay | 48 h | IC50=0.20893 μM | 17696516 | ||
| RXF393 | Growth Inhibition Assay | 48 h | IC50=0.17783 μM | 17696516 | ||
| IGROVI | Growth Inhibition Assay | 48 h | IC50=0.10965 μM | 17696516 | ||
| BxPc3 | Growth Inhibition Assay | 3 h | IC50=0.31623 μM | 17696516 | ||
| PC3 | Function Assay | 20 nM | 72 h | Induction of senescence in human PC3 cells | 17658777 | |
| UO31 | Cytotoxic Assay | 72 h | IC50=0.1 μM | 17656091 | ||
| DLD1 | Cytotoxic Assay | 72 h | IC50=0.1 μM | 17656091 | ||
| UACC62 | Growth Inhibition Assay | 48 h | DMSO | GI50=0.094 μM | 17614292 | |
| LNcaP | Cytotoxic Assay | DMSO | ED50=0.04 μM | 17591444 | ||
| 1A9 | Cytotoxic Assay | DMSO | ED50=0.02 μM | 17591444 | ||
| ZR-751 | Cytotoxic Assay | 72 h | DMSO | IC50=0.04 μM | 17585747 | |
| BGC823 | Cytotoxic Assay | 48 h | IC50=0.35 μM | 17583953 | ||
| 2780AD | Growth Inhibition Assay | 10 μM | Growth inhibition of multidrug-resistant human 2780AD cells with IC50 of 0.298 μM | 17490878 | ||
| KB-VIN | Cytotoxic Assay | 72 h | DMSO | Cytotoxicity against human multidrug-resistant KB-VIN cells with EC50 of 1.7 μM | 17378530 | |
| MeVo | Cytotoxic Assay | 48 h | water | IC50=0.82 μM | 17375902 | |
| CEM/VM1 | Cytotoxic Assay | 72 h | DMSO | Cytotoxicity against teniposide-resistant CEM/VM1 cells with IC50 of 0.55 μM | 17323939 | |
| CEM/VLB100 | Cytotoxic Assay | 72 h | DMSO | Cytotoxicity against CEM/VLB100 cells with IC50 of 3.71 μM | 17323939 | |
| K562I/S9 | Growth Inhibition Assay | 72 h | DMSO | Antiproliferative activity against human multidrug-resistant K562i/S9 cells expressing Pgp with IC50 of 2 μM | 17276690 | |
| 2780AD | Cytotoxic Assay | 10 μM | Cytotoxicity againt multidrug-resistant human 2780AD cells with IC50 of 0.909 μM | 17239597 | ||
| TRAMP-C2H | Growth Inhibition Assay | 72 h | water | GI50=0.0158 μM | 17181166 | |
| TRAMP-C1A | Growth Inhibition Assay | 72 h | water | GI50=0.0204 μM | 17181166 | |
| K562 | Growth Inhibition Assay | 48 h | DMSO/methanol | IC50=0.01 μM | 17181164 | |
| MDA435/LCC6 | Growth Inhibition Assay | 4 d | DMSO | IC50=0.3 μM | 17154505 | |
| MDA-MB-231 | Cytotoxic Assay | 72 h | DMSO | IC50=0.03 μM | 17141923 | |
| A2780 | Growth Inhibition Assay | 1 μM | 4 d | DMSO | Inhibition of doxorubicin-resistant A2780 cell growth with IC50 of 0.061 μM | 17125254 |
| A2780 | Growth Inhibition Assay | 1 μM | 4 d | DMSO | Inhibition of A2780 cell growth with IC50 of 0.003 μM | 17125254 |
| Vero | Cytotoxic Assay | 24 h | DMSO | IC50=0.50119 μM | 17125253 | |
| SK-HEP-1 | Growth Inhibition Assay | 24 h | DMSO | IC50=0.05012 μM | 17125253 | |
| SHP77 | Growth Inhibition Assay | 24 h | DMSO | IC50=0.12589 μM | 17125253 | |
| H9c2 | Growth Inhibition Assay | 24 h | DMSO | IC50=0.01995 μM | 17125253 | |
| AGS | Cytotoxic Assay | IC50=0.31 μM | 17125240 | |||
| HA22T | Cytotoxic Assay | 72 h | DMSO | IC50=0.31 μM | 17107806 | |
| HL60/ADR | Cytotoxic Assay | 1 mM | 72 h | DMSO | Cytotoxicity against adriamycin-resistant HL60/ADR cells expressing MRP1 with IC50 of 2 μM | 17069934 |
| BC | Cytotoxic Assay | IC50=0.288 μM | 16933890 | |||
| Wil2-NS | Growth Inhibition Assay | 96 h | DMSO | IC50=0.02 μM | 16913700 | |
| G361 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.09 μM | 16913700 | |
| C8166 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.02 μM | 16913700 | |
| ACHN | Growth Inhibition Assay | 96 h | DMSO | IC50=0.001 μM | 16913700 | |
| 5637 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.02 μM | 16913700 | |
| MCF7 | Function Assay | 0.5 μg/mL | 24 h | DMSO | Inhibition of p53-mediated transactivation | 16862141 |
| VA13 | Growth Inhibition Assay | 48 h | DMSO | IC50=0.22 μM | 16499322 | |
| MESSA/DX5 | Cytotoxic Assay | Cytotoxicity against multidrug-resistant MES-SA/Dx5 cells with IC50 of 1.56 μM | 16169719 | |||
| MES-SA | Cytotoxic Assay | IC50=0.016 μM | 16169719 | |||
| HSG | Cytotoxic Assay | 24 h | CC50=1.9 μM | 15745812 | ||
| HSC-2 | Cytotoxic Assay | 24 h | CC50=0.68 μM | 15745812 | ||
| NCI-H69 | Cytotoxic Assay | 6 d | IC50=0.0113 μM | 15743178 | ||
| VA13 | Cytotoxic Assay | 48 h | DMSO | IC50=0.38 μM | 15730243 | |
| MeVo | Growth Inhibition Assay | 24 h | water | IC50=0.82 μM | 15715481 | |
| WI38 | Cytotoxic Assay | 48 h | DMSO | IC50=0.3 μM | 15620238 | |
| Rtx6 | Growth Inhibition Assay | 4 h | DMSO | Growth inhibition of ER expressing Rtx6 cells | 15588086 | |
| MEL-28 | Cytotoxic Assay | IC50=0.03 μM | 15225700 | |||
| Col2 | Cytotoxic Assay | 72 h | DMSO | IC50=0.52 μM | 15177438 | |
| U937/GTB | Cytotoxic Assay | 72 h | IC50=0.1 μM | 14987049 | ||
| RPMI8226 | Cytotoxic Assay | 72 h | IC50=0.1 μM | 14987049 | ||
| T47D | Cytotoxic Assay | 72 h | IC50=0.007 μM | 14980672 | ||
| MD-MB468 | Cytotoxic Assay | 72 h | IC50=0.007 μM | 14980672 | ||
| TRAMP-C2G | Growth Inhibition Assay | 72 h | IC50=0.0287 μM | 14971910 | ||
| TRAMP-C2D | Growth Inhibition Assay | 72 h | IC50=0.0465 μM | 14971910 | ||
| MDA-MB-435 | Growth Inhibition Assay | 4 h | DMSO | IC50=0.15 μM | 14971899 | |
| PANC-1 | Growth Inhibition Assay | 72 h | DMSO | GI50=3.5 μM | 14971893 | |
| MEL-28 | Growth Inhibition Assay | 72 h | DMSO | GI50=3.1 μM | 14971893 | |
| LOVO | Growth Inhibition Assay | 72 h | DMSO | GI50=0.01 μM | 14971893 | |
| LNcaP | Growth Inhibition Assay | 72 h | DMSO | GI50=0.02 μM | 14971893 | |
| IGROV | Growth Inhibition Assay | 72 h | DMSO | GI50=4.4 μM | 14971893 | |
| HeLa | Growth Inhibition Assay | 72 h | DMSO | GI50=0.01 μM | 14971893 | |
| DU145 | Growth Inhibition Assay | 72 h | DMSO | GI50=0.02 μM | 14971893 | |
| DuPRO | Cytotoxic Assay | EC50=1.8 μM | 14667208 | |||
| SF268 | Cytotoxic Assay | GI50=8.5 × 10-7 μg/mL | 14640532 | |||
| MCF7 | Cytotoxic Assay | GI50=8.0 × 10-7 μg/mL | 14640532 | |||
| H460 | Cytotoxic Assay | GI50=3.0 × 10-7 μg/mL | 14640532 | |||
| HT1080 | Growth Inhibition Assay | 72 h | DMSO | IC50=0.059 μM | 14640513 | |
| Neuro2A | Function Assay | 3 ug/mL | 24 h | Neuritogenic activity assessed as morphological changes | 14575440 | |
| RXF944L | Growth Inhibition Assay | 72 h | DMSO | IC50=0.06 μM | 12852768 | |
| LXF629L | Growth Inhibition Assay | 72 h | DMSO | IC50=0.07 μM | 12852768 | |
| LXF529L | Growth Inhibition Assay | 72 h | DMSO | IC50=0.03 μM | 12852768 | |
| 401NL | Growth Inhibition Assay | 72 h | DMSO | IC50=0.01 μM | 12852768 | |
| L2987 | Cytotoxic Assay | IC50=0.3 μM | 12798317 | |||
| P388 | Growth Inhibition Assay | 72 h | IC50=0.015 μM | 12620081 | ||
| Jurkat | Growth Inhibition Assay | 72 h | IC50=0.0096 μM | 12620081 | ||
| 8226/S | Cytotoxic Assay | 72 h | DMSO | Cytotoxity against 8226/S | 12570378 | |
| 8226/DOX1V | Cytotoxic Assay | 72 h | DMSO | Cytotoxity against 8226/DOX1V | 12570378 | |
| LXFL529 | Growth Inhibition Assay | 36 h | IC50=0.04 μM | 12459020 | ||
| L1210 | Cytotoxic Assay | 48 h | DMSO | IC50=0.025 μM | 12443763 | |
| HET-SR-2SC-LNM | Growth Inhibition Assay | 72 h | deionized water | IC50=0.052 μM | 12431060 | |
| 2SC/20 | Growth Inhibition Assay | 72 h | deionized water | IC50=0.62 μM | 12431060 | |
| Wil-NS | Growth Inhibition Assay | 96 h | DMSO | IC50=0.02 μM | 12431049 | |
| Raji | Growth Inhibition Assay | 96 h | DMSO | IC50=0.03 μM | 12431049 | |
| Hep-2 | Growth Inhibition Assay | 96 h | DMSO | IC50=0.04 μM | 12431049 | |
| SK-MES-1 | Cytotoxic Assay | 6 d | DMSO | IC50=0.03 μM | 12431048 | |
| SKMEL-28 | Cytotoxic Assay | 6 d | DMSO | IC50=0.06 μM | 12431048 | |
| CRL7065 | Cytotoxic Assay | 6 d | DMSO | IC50=0.5 μM | 12431048 | |
| UMUC3 | Cytotoxic Assay | IC50=0.0092 μM | 12392727 | |||
| U251 | Cytotoxic Assay | IC50=0.09 μM | 12039588 | |||
| H69P | Growth Inhibition Assay | 4 d | IC50=0.027 μM | 11806725 | ||
| H69/LX4 | Growth Inhibition Assay | 4 d | Inhibitory activity against drug resistant H69/LX4 cell overexpressing P-glycoprotein with IC50 of 0.137 μM | 11806725 | ||
| H69P | Cytotoxic Assay | 5-6 d | IC50=0.0273 μM | 11806724 | ||
| COR-L23 | Cytotoxic Assay | 5-6 d | IC50=0.0201 μM | 11806724 | ||
| HCT116 | Growth Inhibition Assay | GI50=0.023 μM | 11755363 | |||
| SKVLB1 | Cytotoxic Assay | 72 h | DMSO | Cytotoxicity against human multidrug-resistant SKVLB1 cell with IC50 of 2.2 μM | 11754602 | |
| UACC62 | Cytotoxic Assay | 3-5 m | DMSO | GI50=0.094 μM | 11678649 | |
| U87 | Cytotoxic Assay | 10 μM | DMSO | Cytotoxicity against U87 resistant cell with IC50 of 0.14 μM | 11606141 | |
| U87 | Cytotoxic Assay | 10 μM | DMSO | Cytotoxicity against U87 sensitive cell with IC50 of 0.06 μM | 11606141 | |
| U373 | Cytotoxic Assay | 10 μM | DMSO | Cytotoxicity against U373 sensitive cell with IC50 of 0.01 μM | 11606141 | |
| T24 | Cytotoxic Assay | 10 μM | DMSO | Cytotoxicity against T24 sensitive cell with IC50 of 0.02 μM | 11606141 | |
| LoVo | Cytotoxic Assay | 10 μM | DMSO | Cytotoxicity against LoVo sensitive cell with IC50 of 0.008 μM | 11606141 | |
| J82 | Cytotoxic Assay | 10 μM | DMSO | Cytotoxicity against J82 sensitive cell with IC50 of 0.03 μM | 11606141 | |
| CNS | Growth Inhibition Assay | 48 h | GI50=0.064 μM | 11473418 | ||
| RENCA | Growth Inhibition Assay | 48 h | IC50<0.01 μM | 11454467 | ||
| CH1 | Cytotoxic Assay | 96 h | DMSO | IC50=0.0063 μM | 11123989 | |
| UA375 | Cytotoxic Assay | 24 h | DMSO | IC50=0.112 μM | 10956214 | |
| OVCAR-3 | Cytotoxic Assay | 24 h | DMSO | IC50=0.035 μM | 10956214 | |
| D40 | Cytotoxic Assay | 24 h | DMSO | IC50=1.172 μM | 10956214 | |
| L1210 | Cytotoxic Assay | IC50=0.025 μM | 10890167 | |||
| ZR-75-1 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against ZR-75-1 cell (ER+,pgr+,mutant p53) with IC50 of 0.009 μM | 10780913 |
| T47D | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against T47D cell (ER+,mutant p53) with IC50 of 0.007 μM | 10780913 |
| SKBR-3 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against SKBR-3 cell (ER-amplified erB2,mutant p53) with IC50 of 0.02 μM | 10780913 |
| NCI-H647 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against NCI-H647 cell (mutant p53) with IC50 of 0.001 μM | 10780913 |
| NCI-H358 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against NCI-H358 cell (mutant p53) with IC50 of 0.13 μM | 10780913 |
| NCI-H322 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against NCI-H322 cell (mutant p53) with IC50 of 0.03 μM | 10780913 |
| NCI-H226 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against NCI-H226 cell (mutant p53) with IC50 of 0.008 μM | 10780913 |
| MDA-468 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against MDA-468 cell (ER-, amplified EGFR, mutant p53) with IC50 of 0.005 μM | 10780913 |
| MDA-231 | Cytotoxic Assay | 100 μM | 48 h | Saline | Cytotoxicity against MDA-231 cell (ER-,EGFR+,mutant p53) with IC50 of 0.006 μM | 10780913 |
| H460pv8 | Cytotoxic Assay | 100 μM | 48 h | Saline | IC50=0.004 μM | 10780913 |
| H460pv8/eto | Cytotoxic Assay | 100 μM | 48 h | Saline | IC50=0.02 μM | 10780913 |
| U937 | Function Assay | 24 h | DMSO | Inhibits incorporation of [3H]thymidine with IC50 of 0.1 μM | 10780911 | |
| T24 | Growth Inhibition Assay | IC50=0.64 μM | 10698436 | |||
| PC14 | Growth Inhibition Assay | IC50=1.9 μM | 10698436 | |||
| MKN-1 | Growth Inhibition Assay | IC50=0.64 μM | 10698436 | |||
| HMV-1 | Growth Inhibition Assay | IC50=0.28 μM | 10698436 | |||
| P388 | Cytotoxic Assay | 72 h | GI50=0.012 μM | 10691710 | ||
| 3Y1 | Cytotoxic Assay | 72 h | GI50=0.0038 μM | 10691710 | ||
| PACA-2 | Cytotoxic Assay | 6 d | ED50=0.0063 μM | 10498214 | ||
| K562R | Cytotoxic Assay | 72 h | IC50=0.0156 μM | 10479282 | ||
| DU145 | Cytotoxic Assay | 72 h | IC50=0.0528 μM | 10479282 | ||
| MEL-AC | Cytotoxic Assay | 100 μM | IC50=1.2 μM | 10476861 | ||
| HTB-54 | Cytotoxic Assay | 100 μM | IC50=1.1 μM | 10476861 | ||
| KB | Growth Inhibition Assay | 4 d | DMSO | Antiproliferative activity against drug-resistant KBV20C with IC50 of 0.4 μM | 10411476 | |
| KB | Growth Inhibition Assay | 4 d | DMSO | Antiproliferative activity against drug-resistant KBMDR with IC50 of 0.5 μM | 10411476 | |
| KB | Growth Inhibition Assay | 4 d | DMSO | Antiproliferative activity against drug-resistant KBCampwith IC50 of 0.06 μM | 10411476 | |
| KB | Growth Inhibition Assay | 4 d | DMSO | Antiproliferative activity against drug-resistant KB7D with IC50 of 0.65 μM | 10411476 | |
| CHO-K1 | Growth Inhibition Assay | 4 d | DMSO | IC50=0.4 μM | 10411476 | |
| CCRF-SB | Growth Inhibition Assay | 4 d | DMSO | IC50=0.01 μM | 10411476 | |
| MDA-MB-361 | Cytotoxic Assay | 1 h | DMSO | Cytotoxicity against MDA MB 361 expressing carboxypeptidase stCPG2 with IC50 of 0.018 μM | 10395490 | |
| MDA-MB-361 | Cytotoxic Assay | 1 h | DMSO | Cytotoxicity against MDA-MB-361 expressing LacZ with IC50 of 0.012 μM | 10395490 | |
| SF-295 | Cytotoxic Assay | 72 h | IC50=0.035 μM | 9873661 | ||
| HOP62 | Cytotoxic Assay | 72 h | IC50=0.004 μM | 9873661 | ||
| PC14 | Cytotoxic Assay | 4 d | DMSO | IC50=0.29 μM | 9822542 | |
| Panc02 | Growth Inhibition Assay | 10 μM | 6 d | IC50=0.065 μM | 9526570 | |
| CHO | Growth Inhibition Assay | 10 μM | 6 d | IC50=0.06 μM | 9526570 | |
| CA755 | Growth Inhibition Assay | 10 μM | 6 d | IC50=0.014 μM | 9526570 | |
| MCF7/VP16 | Cytotoxic Assay | 50 μg/mL | 72 h | IC50=14.2 μM | 9022792 | |
| SKVLB | Cytotoxic Assay | 72 h | IC50=6.54 μM | 9003520 | ||
| C6 | Cytotoxic Assay | 2.5 mg/mL | 5 h | DMSO | Cytotoxicity assessed as cell release | 8988604 |
| C6 | Function Assay | 2.5 mg/mL | 4 h | DMSO | Inhibition of tubulin polymerization | 8988604 |
| C6 | Cytotoxic Assay | 2.5 mg/mL | 72 h | DMSO | Cytotoxicity against rat C6 cells at 50 ug/mL to 2.5 mg/mL | 8988604 |
| MCF | Function Assay | Reversal of P-gp-mediated multidrug resistance assessed as enhancement in cytotoxicity of adriamycin | 8984151 | |||
| WiDr | Cytotoxic Assay | Activity against sensitive WiDr cell with IC50 of 0.053 μM | 8960558 | |||
| MXF7 | Cytotoxic Assay | Antitumor activity against doxorubicin-resistant MXF7 with IC50 of 1.172 μM | 8960558 | |||
| MXF7 | Cytotoxic Assay | Antitumor activity against MXF7 with IC50 of 0.028 μM | 8960558 | |||
| MXF7 | Cytotoxic Assay | Antitumor activity against sensitive MXF7 with IC50 of 0.028 μM | 8960558 | |||
| A2780-DX5 | Cytotoxic Assay | Cytotoxicity against doxorubicin-resistant A2780-DX5 with IC50 of 0.357 μM | 8831755 | |||
| A2780-CP3 | Cytotoxic Assay | Cytotoxicity against A2780-CP3 with IC50 of 0.056 μM | 8831755 | |||
| A2780-C25 | Cytotoxic Assay | Cytotoxicity against A2780-C25 with IC50 of 0.063 μM | 8831755 | |||
| AUC375 | Growth Inhibition Assay | IC50=0.112 μM | 8648600 | |||
| SKVLB | Cytotoxic Assay | IC50=5.6 μM | 8576914 | |||
| EAC | Cytotoxic Assay | 72 h | IC50=0.35 μM | 8350087 | ||
| C38 | Cytotoxic Assay | IC50=0.01 μM | 8057291 | |||
| LOX | Cytotoxic Assay | IC50=0.042 μM | 7853331 | |||
| H2981 | Cytotoxic Assay | IC50=0.5 μM | 7731023 | |||
| EMT6 | Growth Inhibition Assay | 0.5 μM | 1 h | Inhibition of proliferation by measuring for surviving fractions with ED50 of 0.0001 μM | 7452674 | |
| HT-29 | Growth Inhibition Assay | 0.01 μg | 48 h | Antiproliferative activity | 2778449 | |
| KBM-3 | Growth Inhibition Assay | IC50=0.026 μM | 1995877 | |||
| MLM | Cytotoxic Assay | ED50=0.13 μM | 1875350 | |||
| SKMEL-5 | Growth Inhibition Assay | ED50=3.2 μM | 1613753 | |||
| T-MT4 | Growth Inhibition Assay | ID50=0.01 μM | 1548681 | |||
| T-H9 | Growth Inhibition Assay | ID50=0.01 μM | 1548681 | |||
| T-C8166 | Growth Inhibition Assay | ID50=0.01 μM | 1548681 | |||
| H9 | Growth Inhibition Assay | Inhibition of H9 cells chronically infected with the HIV-1 (H9/IIIB) with ID50 of 0.01 μM | 1548681 | |||
| N417 | Growth Inhibition Assay | IC50=0.23 μM | 1479585 | |||
| H69 | Growth Inhibition Assay | IC50=0.87 μM | 1479585 | |||
| MCF7/ADR | Growth Inhibition Assay | IC50=3.1 μM | 1447730 | |||
| 8226/ADR | Growth Inhibition Assay | IC50=0.56 μM | 1447730 | |||
| 8226 | Growth Inhibition Assay | IC50=0.033 μM | 1447730 | |||
| CHO | Growth Inhibition Assay | IC50=0.06 μM | 1354750 | |||
| Yoshida sarcoma | Function Assay | Inhibition of [3H]TdR incorporation with IC50 of 0.02 μM | 1322986 | |||
| L1210 | Function Assay | Inhibition of RNA synthesis with ED50 of 0.58 μM | 490536 | |||
| A431 | Cytotoxic Assay | IC50=0.00135 μM | 24890652 | |||
| HT-29 | Function Assay | 25 μM | 24 h | Inhibition of MRP3-mediated drug resistance in doxorubicin-resistant human HT-29 cells assessed as increase in drug accumulation | 24900337 | |
| HT-29 | Function Assay | 5 μM | 24 h | Inhibition of MRP3 in doxorubicin-resistant human HT-29 cells assessed as nitration of tyrosine residues | 24900337 | |
| HT-29 | Function Assay | 5 μM | 1 h | Inhibition of MRP3-mediated drug resistance in doxorubicin-resistant human HT-29 cells assessed as increase in drug accumulation | 24900337 | |
| HT-29 | Function Assay | 5 μM | 1 h | Induction of nitrite production in doxorubicin-resistant human HT-29 cells | 24900337 | |
| NAR | Function Assay | 5 μM | 2 h | Drug uptake in doxorubicin-resistant human NAR cell plasma membrane | 24900344 | |
| NAR | Cytotoxic Assay | 10 μM | 24 h | Cytotoxicity assessed as induction of cell death | 24900344 | |
| NAR | Cytotoxic Assay | 100 μM | 24 h | Cytotoxicity against doxorubicin-resistant human NAR cells assessed as cell viability | 24900344 | |
| DA-3 | Cytotoxic Assay | 60 μM | 24 h | IC50=4 μM | 24900668 | |
| DA-3 | Function Assay | 10 μM | 10 h | Cellular uptake in mouse DA-3 cell mitochondria | 24900668 | |
| DA-3 | Function Assay | 10 μM | 10 h | Cellular uptake in mouse DA-3 cell lysosome | 24900668 | |
| DA-3 | Function Assay | 10 μM | 10 h | Cellular uptake in mouse DA-3 cell nucleus | 24900668 | |
| YOYO1 | Function Assay | Displacement of YOYO1 from phage lambda DNA with IC50 of 0.6 μM | 24900668 | |||
| KCL22 | Growth Inhibition Assay | 48 h | IC50=1.8 μM | 24941129 | ||
| Saos2 | Growth Inhibition Assay | 40 μM | 24 h | DMSO | IC50=0.33 μM | 24941130 |
| IMR32 | Cytotoxic Assay | 250 μM | 48 h | DMSO | IC50=0.0051 μM | 24996140 |
| NTERA-S-cl-D1 | Growth Inhibition Assay | IC50=1.37 nM | SANGER | |||
| EW-16 | Growth Inhibition Assay | IC50=1.91 nM | SANGER | |||
| HUTU-80 | Growth Inhibition Assay | IC50=2.12 nM | SANGER | |||
| KS-1 | Growth Inhibition Assay | IC50=3.44 nM | SANGER | |||
| HT-144 | Growth Inhibition Assay | IC50=3.51 nM | SANGER | |||
| MFH-ino | Growth Inhibition Assay | IC50=3.99 nM | SANGER | |||
| LC-2 | Growth Inhibition Assay | IC50=5.07 nM | SANGER | |||
| SW982 | Growth Inhibition Assay | IC50=6.05 nM | SANGER | |||
| ES4 | Growth Inhibition Assay | IC50=6.11 nM | SANGER | |||
| KGN | Growth Inhibition Assay | IC50=6.34 nM | SANGER | |||
| RH-1 | Growth Inhibition Assay | IC50=6.73 nM | SANGER | |||
| IGROV-1 | Growth Inhibition Assay | IC50=7.2 nM | SANGER | |||
| KYSE-270 | Growth Inhibition Assay | IC50=7.21 nM | SANGER | |||
| TE-8 | Growth Inhibition Assay | IC50=7.5 nM | SANGER | |||
| Daoy | Growth Inhibition Assay | IC50=8.07 nM | SANGER | |||
| REH | Growth Inhibition Assay | IC50=8.11 nM | SANGER | |||
| 786-0 | Growth Inhibition Assay | IC50=8.47 nM | SANGER | |||
| A375 | Growth Inhibition Assay | IC50=9.17 nM | SANGER | |||
| EW-13 | Growth Inhibition Assay | IC50=9.63 nM | SANGER | |||
| MKN1 | Growth Inhibition Assay | IC50=9.86 nM | SANGER | |||
| MV-4-11 | Growth Inhibition Assay | IC50=10.03 nM | SANGER | |||
| ESS-1 | Growth Inhibition Assay | IC50=11.09 nM | SANGER | |||
| PSN1 | Growth Inhibition Assay | IC50=11.22 nM | SANGER | |||
| MCF7 | Growth Inhibition Assay | IC50=11.65 nM | SANGER | |||
| A427 | Growth Inhibition Assay | IC50=11.81 nM | SANGER | |||
| NCI-H1651 | Growth Inhibition Assay | IC50=11.83 nM | SANGER | |||
| LCLC-97TM1 | Growth Inhibition Assay | IC50=11.83 nM | SANGER | |||
| DU-4475 | Growth Inhibition Assay | IC50=12.1 nM | SANGER | |||
| SK-LU-1 | Growth Inhibition Assay | IC50=12.54 nM | SANGER | |||
| NCI-H460 | Growth Inhibition Assay | IC50=12.7 nM | SANGER | |||
| A204 | Growth Inhibition Assay | IC50=12.88 nM | SANGER | |||
| AGS | Growth Inhibition Assay | IC50=13.27 nM | SANGER | |||
| NCI-H2228 | Growth Inhibition Assay | IC50=13.48 nM | SANGER | |||
| HGC-27 | Growth Inhibition Assay | IC50=13.56 nM | SANGER | |||
| RPMI-2650 | Growth Inhibition Assay | IC50=13.69 nM | SANGER | |||
| ES1 | Growth Inhibition Assay | IC50=13.72 nM | SANGER | |||
| MEL-JUSO | Growth Inhibition Assay | IC50=14.5 nM | SANGER | |||
| HT-1080 | Growth Inhibition Assay | IC50=14.82 nM | SANGER | |||
| OCUB-M | Growth Inhibition Assay | IC50=15.15 nM | SANGER | |||
| 23132-87 | Growth Inhibition Assay | IC50=15.42 nM | SANGER | |||
| KYSE-510 | Growth Inhibition Assay | IC50=15.48 nM | SANGER | |||
| ST486 | Growth Inhibition Assay | IC50=15.53 nM | SANGER | |||
| 639-V | Growth Inhibition Assay | IC50=15.91 nM | SANGER | |||
| COLO-679 | Growth Inhibition Assay | IC50=15.97 nM | SANGER | |||
| CTV-1 | Growth Inhibition Assay | IC50=16.07 nM | SANGER | |||
| BFTC-905 | Growth Inhibition Assay | IC50=16.18 nM | SANGER | |||
| SW954 | Growth Inhibition Assay | IC50=16.93 nM | SANGER | |||
| CAL-51 | Growth Inhibition Assay | IC50=17.32 nM | SANGER | |||
| SW1710 | Growth Inhibition Assay | IC50=17.4 nM | SANGER | |||
| HCC2998 | Growth Inhibition Assay | IC50=17.44 nM | SANGER | |||
| A2058 | Growth Inhibition Assay | IC50=17.65 nM | SANGER | |||
| HCE-4 | Growth Inhibition Assay | IC50=18.07 nM | SANGER | |||
| MEL-HO | Growth Inhibition Assay | IC50=18.3 nM | SANGER | |||
| SNG-M | Growth Inhibition Assay | IC50=18.32 nM | SANGER | |||
| LS-123 | Growth Inhibition Assay | IC50=18.46 nM | SANGER | |||
| NALM-6 | Growth Inhibition Assay | IC50=18.63 nM | SANGER | |||
| NCI-H810 | Growth Inhibition Assay | IC50=18.76 nM | SANGER | |||
| YKG-1 | Growth Inhibition Assay | IC50=18.77 nM | SANGER | |||
| 647-V | Growth Inhibition Assay | IC50=18.81 nM | SANGER | |||
| ONS-76 | Growth Inhibition Assay | IC50=18.91 nM | SANGER | |||
| 697 | Growth Inhibition Assay | IC50=19.09 nM | SANGER | |||
| RS4-11 | Growth Inhibition Assay | IC50=19.14 nM | SANGER | |||
| 8505C | Growth Inhibition Assay | IC50=19.56 nM | SANGER | |||
| SK-UT-1 | Growth Inhibition Assay | IC50=19.96 nM | SANGER | |||
| DOHH-2 | Growth Inhibition Assay | IC50=19.99 nM | SANGER | |||
| NCCIT | Growth Inhibition Assay | IC50=20.13 nM | SANGER | |||
| LCLC-103H | Growth Inhibition Assay | IC50=20.54 nM | SANGER | |||
| CAL-12T | Growth Inhibition Assay | IC50=20.65 nM | SANGER | |||
| QIMR-WIL | Growth Inhibition Assay | IC50=20.72 nM | SANGER | |||
| SBC-5 | Growth Inhibition Assay | IC50=20.74 nM | SANGER | |||
| 769-P | Growth Inhibition Assay | IC50=20.76 nM | SANGER | |||
| A431 | Growth Inhibition Assay | IC50=20.87 nM | SANGER | |||
| KE-37 | Growth Inhibition Assay | IC50=21.05 nM | SANGER | |||
| ES8 | Growth Inhibition Assay | IC50=21.07 nM | SANGER | |||
| 22RV1 | Growth Inhibition Assay | IC50=21.24 nM | SANGER | |||
| LOUCY | Growth Inhibition Assay | IC50=21.32 nM | SANGER | |||
| J82 | Growth Inhibition Assay | IC50=21.34 nM | SANGER | |||
| MHH-ES-1 | Growth Inhibition Assay | IC50=21.58 nM | SANGER | |||
| MG-63 | Growth Inhibition Assay | IC50=22.08 nM | SANGER | |||
| 8-MG-BA | Growth Inhibition Assay | IC50=22.12 nM | SANGER | |||
| BPH-1 | Growth Inhibition Assay | IC50=22.12 nM | SANGER | |||
| RKO | Growth Inhibition Assay | IC50=22.28 nM | SANGER | |||
| HMV-II | Growth Inhibition Assay | IC50=22.6 nM | SANGER | |||
| NCI-H727 | Growth Inhibition Assay | IC50=22.79 nM | SANGER | |||
| MOLT-4 | Growth Inhibition Assay | IC50=22.86 nM | SANGER | |||
| NCI-H2342 | Growth Inhibition Assay | IC50=22.91 nM | SANGER | |||
| MZ1-PC | Growth Inhibition Assay | IC50=23.81 nM | SANGER | |||
| TE-15 | Growth Inhibition Assay | IC50=24.14 nM | SANGER | |||
| SR | Growth Inhibition Assay | IC50=24.41 nM | SANGER | |||
| CCRF-CEM | Growth Inhibition Assay | IC50=24.52 nM | SANGER | |||
| SIG-M5 | Growth Inhibition Assay | IC50=25.69 nM | SANGER | |||
| SK-PN-DW | Growth Inhibition Assay | IC50=25.72 nM | SANGER | |||
| SK-HEP-1 | Growth Inhibition Assay | IC50=25.86 nM | SANGER | |||
| SW620 | Growth Inhibition Assay | IC50=26.63 nM | SANGER | |||
| HSC-2 | Growth Inhibition Assay | IC50=26.78 nM | SANGER | |||
| CAL-148 | Growth Inhibition Assay | IC50=26.9 nM | SANGER | |||
| COLO-205 | Growth Inhibition Assay | IC50=26.99 nM | SANGER | |||
| EoL-1-cell | Growth Inhibition Assay | IC50=27.78 nM | SANGER | |||
| KYSE-150 | Growth Inhibition Assay | IC50=27.78 nM | SANGER | |||
| G-361 | Growth Inhibition Assay | IC50=28.88 nM | SANGER | |||
| PANC-03-27 | Growth Inhibition Assay | IC50=28.16 nM | SANGER | |||
| NCI-H2030 | Growth Inhibition Assay | IC50=28.26 nM | SANGER | |||
| NCI-H441 | Growth Inhibition Assay | IC50=28.42 nM | SANGER | |||
| A388 | Growth Inhibition Assay | IC50=28.46 nM | SANGER | |||
| BHY | Growth Inhibition Assay | IC50=28.88 nM | SANGER | |||
| KALS-1 | Growth Inhibition Assay | IC50=29.33 nM | SANGER | |||
| NKM-1 | Growth Inhibition Assay | IC50=29.47 nM | SANGER | |||
| BB30-HNC | Growth Inhibition Assay | IC50=29.85 nM | SANGER | |||
| G-401 | Growth Inhibition Assay | IC50=30.2 nM | SANGER | |||
| EW-7 | Growth Inhibition Assay | IC50=30.63 nM | SANGER | |||
| CAN | Growth Inhibition Assay | IC50=31.12 nM | SANGER | |||
| M059J | Growth Inhibition Assay | IC50=31.37 nM | SANGER | |||
| SK-MES-1 | Growth Inhibition Assay | IC50=31.5 nM | SANGER | |||
| 5637 | Growth Inhibition Assay | IC50=31.87 nM | SANGER | |||
| MKN28 | Growth Inhibition Assay | IC50=31.91 nM | SANGER | |||
| COR-L23 | Growth Inhibition Assay | IC50=31.96 nM | SANGER | |||
| CAL-27 | Growth Inhibition Assay | IC50=32.57 nM | SANGER | |||
| CAL-62 | Growth Inhibition Assay | IC50=32.79 nM | SANGER | |||
| C-33-A | Growth Inhibition Assay | IC50=32.89 nM | SANGER | |||
| RT-112 | Growth Inhibition Assay | IC50=32.92 nM | SANGER | |||
| KU812 | Growth Inhibition Assay | IC50=33.24 nM | SANGER | |||
| C8166 | Growth Inhibition Assay | IC50=33.31 nM | SANGER | |||
| VA-ES-BJ | Growth Inhibition Assay | IC50=33.36 nM | SANGER | |||
| NEC8 | Growth Inhibition Assay | IC50=33.61 nM | SANGER | |||
| EB2 | Growth Inhibition Assay | IC50=33.73 nM | SANGER | |||
| COLO-680N | Growth Inhibition Assay | IC50=33.87 nM | SANGER | |||
| IGR-1 | Growth Inhibition Assay | IC50=33.83 nM | SANGER | |||
| MZ2-MEL | Growth Inhibition Assay | IC50=33.92 nM | SANGER | |||
| MIA-PaCa-2 | Growth Inhibition Assay | IC50=34.24 nM | SANGER | |||
| IMR-5 | Growth Inhibition Assay | IC50=34.68 nM | SANGER | |||
| CHP-212 | Growth Inhibition Assay | IC50=34.96 nM | SANGER | |||
| CCF-STTG1 | Growth Inhibition Assay | IC50=35.5 nM | SANGER | |||
| SK-MEL-30 | Growth Inhibition Assay | IC50=35.61 nM | SANGER | |||
| HLE | Growth Inhibition Assay | IC50=35.8 nM | SANGER | |||
| HOP-62 | Growth Inhibition Assay | IC50=35.88 nM | SANGER | |||
| LB1047-RCC | Growth Inhibition Assay | IC50=35.97 nM | SANGER | |||
| DoTc2-4510 | Growth Inhibition Assay | IC50=36.25 nM | SANGER | |||
| HT-29 | Growth Inhibition Assay | IC50=36.57 nM | SANGER | |||
| HC-1 | Growth Inhibition Assay | IC50=37.32 nM | SANGER | |||
| TE-5 | Growth Inhibition Assay | IC50=37.48 nM | SANGER | |||
| NCI-H1299 | Growth Inhibition Assay | IC50=37.59 nM | SANGER | |||
| CHL-1 | Growth Inhibition Assay | IC50=37.73 nM | SANGER | |||
| NCI-H2122 | Growth Inhibition Assay | IC50=37.9 nM | SANGER | |||
| DEL | Growth Inhibition Assay | IC50=38.44 nM | SANGER | |||
| ES5 | Growth Inhibition Assay | IC50=39.22 nM | SANGER | |||
| CESS | Growth Inhibition Assay | IC50=39.36 nM | SANGER | |||
| NCI-H719 | Growth Inhibition Assay | IC50=39.65 nM | SANGER | |||
| A3-KAW | Growth Inhibition Assay | IC50=40.14 nM | SANGER | |||
| KP-4 | Growth Inhibition Assay | IC50=40.77 nM | SANGER | |||
| NCI-H358 | Growth Inhibition Assay | IC50=41.08 nM | SANGER | |||
| HCC38 | Growth Inhibition Assay | IC50=42.5 nM | SANGER | |||
| MRK-nu-1 | Growth Inhibition Assay | IC50=43.66 nM | SANGER | |||
| NCI-H292 | Growth Inhibition Assay | IC50=43.76 nM | SANGER | |||
| PA-1 | Growth Inhibition Assay | IC50=44.48 nM | SANGER | |||
| CAL-85-1 | Growth Inhibition Assay | IC50=44.52 nM | SANGER | |||
| KYSE-450 | Growth Inhibition Assay | IC50=44.71 nM | SANGER | |||
| IST-MEL1 | Growth Inhibition Assay | IC50=44.84 nM | SANGER | |||
| Ca-Ski | Growth Inhibition Assay | IC50=44.84 nM | SANGER | |||
| OCI-AML2 | Growth Inhibition Assay | IC50=45.05 nM | SANGER | |||
| NUGC-3 | Growth Inhibition Assay | IC50=45.2 nM | SANGER | |||
| HOS | Growth Inhibition Assay | IC50=45.29 nM | SANGER | |||
| NB1 | Growth Inhibition Assay | IC50=45.99 nM | SANGER | |||
| A253 | Growth Inhibition Assay | IC50=46.12 nM | SANGER | |||
| NCI-H209 | Growth Inhibition Assay | IC50=48.73 nM | SANGER | |||
| MC-CAR | Growth Inhibition Assay | IC50=48.74 nM | SANGER | |||
| NCI-H661 | Growth Inhibition Assay | IC50=49.03 nM | SANGER | |||
| SF295 | Growth Inhibition Assay | IC50=49.08 nM | SANGER | |||
| GP5d | Growth Inhibition Assay | IC50=49.12 nM | SANGER | |||
| P12-ICHIKAWA | Growth Inhibition Assay | IC50=49.27 nM | SANGER | |||
| ACHN | Growth Inhibition Assay | IC50=49.34 nM | SANGER | |||
| NCI-H2087 | Growth Inhibition Assay | IC50=49.42 nM | SANGER | |||
| SW48 | Growth Inhibition Assay | IC50=49.72 nM | SANGER | |||
| NCI-H526 | Growth Inhibition Assay | IC50=49.96 nM | SANGER | |||
| Ca9-22 | Growth Inhibition Assay | IC50=50.07 nM | SANGER | |||
| SK-OV-3 | Growth Inhibition Assay | IC50=50.12 nM | SANGER | |||
| KYSE-140 | Growth Inhibition Assay | IC50=50.42 nM | SANGER | |||
| BE-13 | Growth Inhibition Assay | IC50=51.02 nM | SANGER | |||
| ES7 | Growth Inhibition Assay | IC50=51.4 nM | SANGER | |||
| IM-9 | Growth Inhibition Assay | IC50=51.43 nM | SANGER | |||
| A101D | Growth Inhibition Assay | IC50=51.48 nM | SANGER | |||
| CAPAN-1 | Growth Inhibition Assay | IC50=51.76 nM | SANGER | |||
| NCI-H2009 | Growth Inhibition Assay | IC50=51.76 nM | SANGER | |||
| NCI-H522 | Growth Inhibition Assay | IC50=51.93 nM | SANGER | |||
| HuP-T4 | Growth Inhibition Assay | IC50=52.22 nM | SANGER | |||
| BCPAP | Growth Inhibition Assay | IC50=52.5 nM | SANGER | |||
| NCI-H1734 | Growth Inhibition Assay | IC50=53 nM | SANGER | |||
| SK-LMS-1 | Growth Inhibition Assay | IC50=53.22 nM | SANGER | |||
| CGTH-W-1 | Growth Inhibition Assay | IC50=53.46 nM | SANGER | |||
| RPMI-8402 | Growth Inhibition Assay | IC50=53.86 nM | SANGER | |||
| Calu-3 | Growth Inhibition Assay | IC50=55.98 nM | SANGER | |||
| COR-L279 | Growth Inhibition Assay | IC50=56.95 nM | SANGER | |||
| T84 | Growth Inhibition Assay | IC50=57.06 nM | SANGER | |||
| P30-OHK | Growth Inhibition Assay | IC50=57.57 nM | SANGER | |||
| CHP-126 | Growth Inhibition Assay | IC50=59.24 nM | SANGER | |||
| KYSE-180 | Growth Inhibition Assay | IC50=59.75 nM | SANGER | |||
| CAMG | Growth Inhibition Assay | IC50=59.96 nM | SANGER | |||
| EW-1 | Growth Inhibition Assay | IC50=61.35 nM | SANGER | |||
| OVCAR-8 | Growth Inhibition Assay | IC50=61.92 nM | SANGER | |||
| HL-60 | Growth Inhibition Assay | IC50=62.56 nM | SANGER | |||
| 8305C | Growth Inhibition Assay | IC50=63.12 nM | SANGER | |||
| IA-LM | Growth Inhibition Assay | IC50=63.54 nM | SANGER | |||
| HCC2157 | Growth Inhibition Assay | IC50=63.95 nM | SANGER | |||
| CTB-1 | Growth Inhibition Assay | IC50=64019 nM | SANGER | |||
| NCI-H1437 | Growth Inhibition Assay | IC50=64.64 nM | SANGER | |||
| U251 | Growth Inhibition Assay | IC50=64.89 nM | SANGER | |||
| ALL-PO | Growth Inhibition Assay | IC50=65.03 nM | SANGER | |||
| NCI-SNU-1 | Growth Inhibition Assay | IC50=65.86 nM | SANGER | |||
| RPMI-6666 | Growth Inhibition Assay | IC50=65.99 nM | SANGER | |||
| DBTRG-05MG | Growth Inhibition Assay | IC50=67.11 nM | SANGER | |||
| PF-382 | Growth Inhibition Assay | IC50=67.17 nM | SANGER | |||
| GT3TKB | Growth Inhibition Assay | IC50=67.19 nM | SANGER | |||
| SCC-3 | Growth Inhibition Assay | IC50=67.28 nM | SANGER | |||
| LU-134-A | Growth Inhibition Assay | IC50=67.68 nM | SANGER | |||
| CAL-33 | Growth Inhibition Assay | IC50=67.87 nM | SANGER | |||
| MC-IXC | Growth Inhibition Assay | IC50=67.9 nM | SANGER | |||
| CPC-N | Growth Inhibition Assay | IC50=68.4 nM | SANGER | |||
| A2780 | Growth Inhibition Assay | IC50=68.79 nM | SANGER | |||
| Daudi | Growth Inhibition Assay | IC50=68.96 nM | SANGER | |||
| GI-1 | Growth Inhibition Assay | IC50=69.65 nM | SANGER | |||
| SW962 | Growth Inhibition Assay | IC50=69.95 nM | SANGER | |||
| MFE-296 | Growth Inhibition Assay | IC50=70.49 nM | SANGER | |||
| H4 | Growth Inhibition Assay | IC50=71.56 nM | SANGER | |||
| AN3-CA | Growth Inhibition Assay | IC50=72.32 nM | SANGER | |||
| HCC1599 | Growth Inhibition Assay | IC50=72.54 nM | SANGER | |||
| HuCCT1 | Growth Inhibition Assay | IC50=72.93 nM | SANGER | |||
| Calu-1 | Growth Inhibition Assay | IC50=73.6 nM | SANGER | |||
| DU-145 | Growth Inhibition Assay | IC50=73.73 nM | SANGER | |||
| IST-SL2 | Growth Inhibition Assay | IC50=73.81 nM | SANGER | |||
| HSC-3 | Growth Inhibition Assay | IC50=74.53 nM | SANGER | |||
| HCC1806 | Growth Inhibition Assay | IC50=74.96 nM | SANGER | |||
| VM-CUB-1 | Growth Inhibition Assay | IC50=75.36 nM | SANGER | |||
| HSC-4 | Growth Inhibition Assay | IC50=75.76 nM | SANGER | |||
| NH-12 | Growth Inhibition Assay | IC50=76 nM | SANGER | |||
| MDA-MB-468 | Growth Inhibition Assay | IC50=76.18 nM | SANGER | |||
| TUR | Growth Inhibition Assay | IC50=76.32 nM | SANGER | |||
| TE-1 | Growth Inhibition Assay | IC50=76.35 nM | SANGER | |||
| CMK | Growth Inhibition Assay | IC50=77.42 nM | SANGER | |||
| LB996-RCC | Growth Inhibition Assay | IC50=78.45 nM | SANGER | |||
| NCI-H1650 | Growth Inhibition Assay | IC50=78.62 nM | SANGER | |||
| DMS-273 | Growth Inhibition Assay | IC50=78.9 nM | SANGER | |||
| NOS-1 | Growth Inhibition Assay | IC50=79.14 nM | SANGER | |||
| NB69 | Growth Inhibition Assay | IC50=79.2 nM | SANGER | |||
| SUP-T1 | Growth Inhibition Assay | IC50=79.72 nM | SANGER | |||
| A172 | Growth Inhibition Assay | IC50=80.55 nM | SANGER | |||
| TE-12 | Growth Inhibition Assay | IC50=81.06 nM | SANGER | |||
| MKN45 | Growth Inhibition Assay | IC50=81.51 nM | SANGER | |||
| LoVo | Growth Inhibition Assay | IC50=81.82 nM | SANGER | |||
| SW1573 | Growth Inhibition Assay | IC50=82.81 nM | SANGER | |||
| ETK-1 | Growth Inhibition Assay | IC50=83.01 nM | SANGER | |||
| ABC-1 | Growth Inhibition Assay | IC50=83.45 nM | SANGER | |||
| COLO-668 | Growth Inhibition Assay | IC50=84.47 nM | SANGER | |||
| UM-UC-3 | Growth Inhibition Assay | IC50=86.02 nM | SANGER | |||
| BC-1 | Growth Inhibition Assay | IC50=86.76 nM | SANGER | |||
| PANC-10-05 | Growth Inhibition Assay | IC50=88.45 nM | SANGER | |||
| ES3 | Growth Inhibition Assay | IC50=89.48 nM | SANGER | |||
| TE-441-T | Growth Inhibition Assay | IC50=89.82 nM | SANGER | |||
| KM-H2 | Growth Inhibition Assay | IC50=90.68 nM | SANGER | |||
| SH-4 | Growth Inhibition Assay | IC50=92.11 nM | SANGER | |||
| SF268 | Growth Inhibition Assay | IC50=92.42 nM | SANGER | |||
| VMRC-RCZ | Growth Inhibition Assay | IC50=93.26 nM | SANGER | |||
| GI-ME-N | Growth Inhibition Assay | IC50=93.33 nM | SANGER | |||
| JEG-3 | Growth Inhibition Assay | IC50=95.12 nM | SANGER | |||
| J-RT3-T3-5 | Growth Inhibition Assay | IC50=96.31 nM | SANGER | |||
| K5 | Growth Inhibition Assay | IC50=96.9 nM | SANGER | |||
| EFO-27 | Growth Inhibition Assay | IC50=97.44 nM | SANGER | |||
| SW1783 | Growth Inhibition Assay | IC50=97.99 nM | SANGER | |||
| CAL-39 | Growth Inhibition Assay | IC50=98.24 nM | SANGER | |||
| LS-441N | Growth Inhibition Assay | IC50=99.06 nM | SANGER | |||
| G-402 | Growth Inhibition Assay | IC50=99.09 nM | SANGER | |||
| MES-SA | Growth Inhibition Assay | IC50=99.53 nM | SANGER | |||
| FADU | Growth Inhibition Assay | IC50=102.18 nM | SANGER | |||
| DJM-1 | Growth Inhibition Assay | IC50=102.25 nM | SANGER | |||
| SU-DHL-1 | Growth Inhibition Assay | IC50=102.5 nM | SANGER | |||
| KYSE-410 | Growth Inhibition Assay | IC50=103.46 nM | SANGER | |||
| NCI-H1618 | Growth Inhibition Assay | IC50=104.35 nM | SANGER | |||
| AU565 | Growth Inhibition Assay | IC50=104.51 nM | SANGER | |||
| SF126 | Growth Inhibition Assay | IC50=104.58 nM | SANGER | |||
| MMAC-SF | Growth Inhibition Assay | IC50=106.48 nM | SANGER | |||
| ChaGo-K-1 | Growth Inhibition Assay | IC50=107.95 nM | SANGER | |||
| NCI-H596 | Growth Inhibition Assay | IC50=108.01 nM | SANGER | |||
| LXF-289 | Growth Inhibition Assay | IC50=109.24 nM | SANGER | |||
| HCC1569 | Growth Inhibition Assay | IC50=109.34 nM | SANGER | |||
| NCI-H2170 | Growth Inhibition Assay | IC50=109.92 nM | SANGER | |||
| ARH-77 | Growth Inhibition Assay | IC50=109.99 nM | SANGER | |||
| BOKU | Growth Inhibition Assay | IC50=110.87 nM | SANGER | |||
| SJSA-1 | Growth Inhibition Assay | IC50=111.05 nM | SANGER | |||
| SW626 | Growth Inhibition Assay | IC50=111.71 nM | SANGER | |||
| EHEB | Growth Inhibition Assay | IC50=112.59 nM | SANGER | |||
| TE-10 | Growth Inhibition Assay | IC50=112.68 nM | SANGER | |||
| MZ7-mel | Growth Inhibition Assay | IC50=112.84 nM | SANGER | |||
| SAS | Growth Inhibition Assay | IC50=114.45 nM | SANGER | |||
| TK10 | Growth Inhibition Assay | IC50=114.6 nM | SANGER | |||
| SW13 | Growth Inhibition Assay | IC50=115.26 nM | SANGER | |||
| HCC1937 | Growth Inhibition Assay | IC50=115.84 nM | SANGER | |||
| SK-NEP-1 | Growth Inhibition Assay | IC50=116.26 nM | SANGER | |||
| OVCAR-5 | Growth Inhibition Assay | IC50=116.81 nM | SANGER | |||
| SW837 | Growth Inhibition Assay | IC50=117.95 nM | SANGER | |||
| BFTC-909 | Growth Inhibition Assay | IC50=118.48 nM | SANGER | |||
| SW872 | Growth Inhibition Assay | IC50=120.81 nM | SANGER | |||
| SF539 | Growth Inhibition Assay | IC50=121.72 nM | SANGER | |||
| MLMA | Growth Inhibition Assay | IC50=122.08 nM | SANGER | |||
| BL-41 | Growth Inhibition Assay | IC50=122.22 nM | SANGER | |||
| LB647-SCLC | Growth Inhibition Assay | IC50=123.35 nM | SANGER | |||
| NCI-H1703 | Growth Inhibition Assay | IC50=126.42 nM | SANGER | |||
| KURAMOCHI | Growth Inhibition Assay | IC50=126.76 nM | SANGER | |||
| ATN-1 | Growth Inhibition Assay | IC50=127.7 nM | SANGER | |||
| SK-CO-1 | Growth Inhibition Assay | IC50=129.21 nM | SANGER | |||
| RPMI-7951 | Growth Inhibition Assay | IC50=130.14 nM | SANGER | |||
| NCI-H1648 | Growth Inhibition Assay | IC50=131.94 nM | SANGER | |||
| NCI-H64 | Growth Inhibition Assay | IC50=132.65 nM | SANGER | |||
| HT-3 | Growth Inhibition Assay | IC50=133.04 nM | SANGER | |||
| NCI-H1770 | Growth Inhibition Assay | IC50=133.21 nM | SANGER | |||
| NB13 | Growth Inhibition Assay | IC50=134.55 nM | SANGER | |||
| NCI-H747 | Growth Inhibition Assay | IC50=135.02 nM | SANGER | |||
| ML-2 | Growth Inhibition Assay | IC50=135.16 nM | SANGER | |||
| CA46 | Growth Inhibition Assay | IC50=136.88 nM | SANGER | |||
| ES6 | Growth Inhibition Assay | IC50=137.12 nM | SANGER | |||
| JiyoyeP-2003 | Growth Inhibition Assay | IC50=137.8 nM | SANGER | |||
| NMC-G1 | Growth Inhibition Assay | IC50=139.13 nM | SANGER | |||
| U-698-M | Growth Inhibition Assay | IC50=139.5 nM | SANGER | |||
| LU-99A | Growth Inhibition Assay | IC50=146.55 nM | SANGER | |||
| NCI-H748 | Growth Inhibition Assay | IC50=147.31 nM | SANGER | |||
| SK-MEL-3 | Growth Inhibition Assay | IC50=147.61 nM | SANGER | |||
| SNB75 | Growth Inhibition Assay | IC50=148.65 nM | SANGER | |||
| BB65-RCC | Growth Inhibition Assay | IC50=149.31 nM | SANGER | |||
| NCI-H1355 | Growth Inhibition Assay | IC50=152.24 nM | SANGER | |||
| SCC-25 | Growth Inhibition Assay | IC50=153.73 nM | SANGER | |||
| OAW-42 | Growth Inhibition Assay | IC50=153.84 nM | SANGER | |||
| MONO-MAC-6 | Growth Inhibition Assay | IC50=154.36 nM | SANGER | |||
| WSU-NHL | Growth Inhibition Assay | IC50=154.58 nM | SANGER | |||
| SNU-423 | Growth Inhibition Assay | IC50=155.34 nM | SANGER | |||
| SBC-1 | Growth Inhibition Assay | IC50=156.4 nM | SANGER | |||
| OVCAR-4 | Growth Inhibition Assay | IC50=156.78 nM | SANGER | |||
| NCI-H2347 | Growth Inhibition Assay | IC50=158.34 nM | SANGER | |||
| CAMA-1 | Growth Inhibition Assay | IC50=159.33 nM | SANGER | |||
| GDM-1 | Growth Inhibition Assay | IC50=159.7 nM | SANGER | |||
| KNS-81-FD | Growth Inhibition Assay | IC50=160.32 nM | SANGER | |||
| no-10 | Growth Inhibition Assay | IC50=160.38 nM | SANGER | |||
| NCI-H2052 | Growth Inhibition Assay | IC50=160.47 nM | SANGER | |||
| DB | Growth Inhibition Assay | IC50=161.71 nM | SANGER | |||
| A4-Fuk | Growth Inhibition Assay | IC50=162.48 nM | SANGER | |||
| HCE-T | Growth Inhibition Assay | IC50=164.87 nM | SANGER | |||
| HuP-T3 | Growth Inhibition Assay | IC50=165.55 nM | SANGER | |||
| BC-3 | Growth Inhibition Assay | IC50=165.86 nM | SANGER | |||
| RVH-421 | Growth Inhibition Assay | IC50=169.13 nM | SANGER | |||
| COLO-800 | Growth Inhibition Assay | IC50=171.8 nM | SANGER | |||
| OMC-1 | Growth Inhibition Assay | IC50=172.17 nM | SANGER | |||
| SJRH30 | Growth Inhibition Assay | IC50=172.94 nM | SANGER | |||
| HEL | Growth Inhibition Assay | IC50=175.15 nM | SANGER | |||
| KLE | Growth Inhibition Assay | IC50=175.47 nM | SANGER | |||
| MEG-01 | Growth Inhibition Assay | IC50=176.6 nM | SANGER | |||
| OAW-28 | Growth Inhibition Assay | IC50=178.84 nM | SANGER | |||
| RD | Growth Inhibition Assay | IC50=180.1 nM | SANGER | |||
| HCC2218 | Growth Inhibition Assay | IC50=181.66 nM | SANGER | |||
| OS-RC-2 | Growth Inhibition Assay | IC50=182.49 nM | SANGER | |||
| HEC-1 | Growth Inhibition Assay | IC50=182.83 nM | SANGER | |||
| SCC-4 | Growth Inhibition Assay | IC50=183.71 nM | SANGER | |||
| ME-180 | Growth Inhibition Assay | IC50=186.08 nM | SANGER | |||
| NB10 | Growth Inhibition Assay | IC50=188.09 nM | SANGER | |||
| SCC-15 | Growth Inhibition Assay | IC50=188.24 nM | SANGER | |||
| COR-L105 | Growth Inhibition Assay | IC50=188.92 nM | SANGER | |||
| HT-1376 | Growth Inhibition Assay | IC50=189.01 nM | SANGER | |||
| RPMI-8866 | Growth Inhibition Assay | IC50=192.13 nM | SANGER | |||
| NCI-H2126 | Growth Inhibition Assay | IC50=192.2 nM | SANGER | |||
| KARPAS-422 | Growth Inhibition Assay | IC50=193.41 nM | SANGER | |||
| COLO-741 | Growth Inhibition Assay | IC50=194.58 nM | SANGER | |||
| IPC-298 | Growth Inhibition Assay | IC50=196.21 nM | SANGER | |||
| OE19 | Growth Inhibition Assay | IC50=200.18 nM | SANGER | |||
| NCI-H1694 | Growth Inhibition Assay | IC50=200.26 nM | SANGER | |||
| SW1990 | Growth Inhibition Assay | IC50=201.9 nM | SANGER | |||
| LNCaP-Clone-FGC | Growth Inhibition Assay | IC50=204.47 nM | SANGER | |||
| LB831-BLC | Growth Inhibition Assay | IC50=206.25 nM | SANGER | |||
| HCT-116 | Growth Inhibition Assay | IC50=207.39 nM | SANGER | |||
| BxPC-3 | Growth Inhibition Assay | IC50=210.96 nM | SANGER | |||
| HT-1197 | Growth Inhibition Assay | IC50=212.58 nM | SANGER | |||
| GB-1 | Growth Inhibition Assay | IC50=212.73 nM | SANGER | |||
| RPMI-8226 | Growth Inhibition Assay | IC50=213.37 nM | SANGER | |||
| L-540 | Growth Inhibition Assay | IC50=213.49 nM | SANGER | |||
| NCI-H1882 | Growth Inhibition Assay | IC50=215 nM | SANGER | |||
| A673 | Growth Inhibition Assay | IC50=215.79 nM | SANGER | |||
| NCI-H650 | Growth Inhibition Assay | IC50=217.61 nM | SANGER | |||
| KM12 | Growth Inhibition Assay | IC50=218.15 nM | SANGER | |||
| KP-N-YS | Growth Inhibition Assay | IC50=218.4 nM | SANGER | |||
| COLO-684 | Growth Inhibition Assay | IC50=219.79 nM | SANGER | |||
| MHH-NB-11 | Growth Inhibition Assay | IC50=220.22 nM | SANGER | |||
| HO-1-N-1 | Growth Inhibition Assay | IC50=222.83 nM | SANGER | |||
| NOMO-1 | Growth Inhibition Assay | IC50=224.43 nM | SANGER | |||
| KYSE-70 | Growth Inhibition Assay | IC50=224.66 nM | SANGER | |||
| NCI-H838 | Growth Inhibition Assay | IC50=224.73 nM | SANGER | |||
| NY | Growth Inhibition Assay | IC50=228.49 nM | SANGER | |||
| NCI-H2196 | Growth Inhibition Assay | IC50=229.15 nM | SANGER | |||
| FTC-133 | Growth Inhibition Assay | IC50=229.37 nM | SANGER | |||
| MHH-PREB-1 | Growth Inhibition Assay | IC50=229.82 nM | SANGER | |||
| SK-MEL-2 | Growth Inhibition Assay | IC50=230.3 nM | SANGER | |||
| Detroit562 | Growth Inhibition Assay | IC50=230.72 nM | SANGER | |||
| NCI-H2405 | Growth Inhibition Assay | IC50=234.1 nM | SANGER | |||
| NCI-H2081 | Growth Inhibition Assay | IC50=234.27 nM | SANGER | |||
| LB2241-RCC | Growth Inhibition Assay | IC50=235.11 nM | SANGER | |||
| SNU-387 | Growth Inhibition Assay | IC50=236.08 nM | SANGER | |||
| PC-14 | Growth Inhibition Assay | IC50=236.12 nM | SANGER | |||
| CAS-1 | Growth Inhibition Assay | IC50=238.31 nM | SANGER | |||
| TE-11 | Growth Inhibition Assay | IC50=240.88 nM | SANGER | |||
| LC4-1 | Growth Inhibition Assay | IC50=240.89 nM | SANGER | |||
| HH | Growth Inhibition Assay | IC50=241.46 nM | SANGER | |||
| JVM-2 | Growth Inhibition Assay | IC50=242.17 nM | SANGER | |||
| KNS-42 | Growth Inhibition Assay | IC50=242.67 nM | SANGER | |||
| T-24 | Growth Inhibition Assay | IC50=245.48 nM | SANGER | |||
| D-392MG | Growth Inhibition Assay | IC50=248.5 nM | SANGER | |||
| KOSC-2 | Growth Inhibition Assay | IC50=248.71 nM | SANGER | |||
| D-283MED | Growth Inhibition Assay | IC50=250.13 nM | SANGER | |||
| BV-173 | Growth Inhibition Assay | IC50=250.77 nM | SANGER | |||
| EW-18 | Growth Inhibition Assay | IC50=250.9 nM | SANGER | |||
| MS-1 | Growth Inhibition Assay | IC50=252.53 nM | SANGER | |||
| Calu-6 | Growth Inhibition Assay | IC50=253.55 nM | SANGER | |||
| GR-ST | Growth Inhibition Assay | IC50=255.42 nM | SANGER | |||
| GAK | Growth Inhibition Assay | IC50=256.91 nM | SANGER | |||
| TE-9 | Growth Inhibition Assay | IC50=257.47 nM | SANGER | |||
| HuH-7 | Growth Inhibition Assay | IC50=258.44 nM | SANGER | |||
| KYSE-520 | Growth Inhibition Assay | IC50=260.77 nM | SANGER | |||
| NCI-H520 | Growth Inhibition Assay | IC50=266.61 nM | SANGER | |||
| HD-MY-Z | Growth Inhibition Assay | IC50=272.79 nM | SANGER | |||
| SW756 | Growth Inhibition Assay | IC50=274.78 nM | SANGER | |||
| NB12 | Growth Inhibition Assay | IC50=277.12 nM | SANGER | |||
| EW-3 | Growth Inhibition Assay | IC50=279.89 nM | SANGER | |||
| AM-38 | Growth Inhibition Assay | IC50=281.52 nM | SANGER | |||
| NCI-H23 | Growth Inhibition Assay | IC50=283.92 nM | SANGER | |||
| GOTO | Growth Inhibition Assay | IC50=285.56 nM | SANGER | |||
| NCI-H1693 | Growth Inhibition Assay | IC50=290.32 nM | SANGER | |||
| MOLT-16 | Growth Inhibition Assay | IC50=290.5 nM | SANGER | |||
| HOP-92 | Growth Inhibition Assay | IC50=290.7 nM | SANGER | |||
| MDA-MB-361 | Growth Inhibition Assay | IC50=292.65 nM | SANGER | |||
| Ramos-2G6-4C10 | Growth Inhibition Assay | IC50=294.55 nM | SANGER | |||
| CAL-54 | Growth Inhibition Assay | IC50=300.73 nM | SANGER | |||
| D-263MG | Growth Inhibition Assay | IC50=305.52 nM | SANGER | |||
| SKG-IIIa | Growth Inhibition Assay | IC50=311.14 nM | SANGER | |||
| HAL-01 | Growth Inhibition Assay | IC50=313.44 nM | SANGER | |||
| LAN-6 | Growth Inhibition Assay | IC50=315.11 nM | SANGER | |||
| NCI-H1304 | Growth Inhibition Assay | IC50=321.17 nM | SANGER | |||
| no-11 | Growth Inhibition Assay | IC50=321.64 nM | SANGER | |||
| IST-MES1 | Growth Inhibition Assay | IC50=323.35 nM | SANGER | |||
| L-363 | Growth Inhibition Assay | IC50=324.43 nM | SANGER | |||
| EM-2 | Growth Inhibition Assay | IC50=325.41 nM | SANGER | |||
| DV-90 | Growth Inhibition Assay | IC50=329.45 nM | SANGER | |||
| Saos-2 | Growth Inhibition Assay | IC50=329.94 nM | SANGER | |||
| UMC-11 | Growth Inhibition Assay | IC50=331.28 nM | SANGER | |||
| Raji | Growth Inhibition Assay | IC50=334.64 nM | SANGER | |||
| RXF393 | Growth Inhibition Assay | IC50=335.43 nM | SANGER | |||
| EW-11 | Growth Inhibition Assay | IC50=341.56 nM | SANGER | |||
| BL-70 | Growth Inhibition Assay | IC50=342.01 nM | SANGER | |||
| LB373-MEL-D | Growth Inhibition Assay | IC50=344.64 nM | SANGER | |||
| LU-139 | Growth Inhibition Assay | IC50=344.92 nM | SANGER | |||
| U-87-MG | Growth Inhibition Assay | IC50=346.04 nM | SANGER | |||
| LU-65 | Growth Inhibition Assay | IC50=346.4 nM | SANGER | |||
| U031 | Growth Inhibition Assay | IC50=353.87 nM | SANGER | |||
| HN | Growth Inhibition Assay | IC50=354.27 nM | SANGER | |||
| MHH-CAL-2 | Growth Inhibition Assay | IC50=359.21 nM | SANGER | |||
| NCI-H510A | Growth Inhibition Assay | IC50=359.59 nM | SANGER | |||
| NB5 | Growth Inhibition Assay | IC50=359.81 nM | SANGER | |||
| KG-1 | Growth Inhibition Assay | IC50=361.52 nM | SANGER | |||
| EFO-21 | Growth Inhibition Assay | IC50=361.85 nM | SANGER | |||
| UACC-257 | Growth Inhibition Assay | IC50=362.05 nM | SANGER | |||
| SW1417 | Growth Inhibition Assay | IC50=363.23 nM | SANGER | |||
| NCI-H1417 | Growth Inhibition Assay | IC50=370.04 nM | SANGER | |||
| DSH1 | Growth Inhibition Assay | IC50=372.72 nM | SANGER | |||
| CAKI-1 | Growth Inhibition Assay | IC50=375.48 nM | SANGER | |||
| SIMA | Growth Inhibition Assay | IC50=381.86 nM | SANGER | |||
| LS-1034 | Growth Inhibition Assay | IC50=384.51 nM | SANGER | |||
| K-562 | Growth Inhibition Assay | IC50=385.8 nM | SANGER | |||
| LOXIMVI | Growth Inhibition Assay | IC50=389.19 nM | SANGER | |||
| MC116 | Growth Inhibition Assay | IC50=391.41 nM | SANGER | |||
| MPP-89 | Growth Inhibition Assay | IC50=392.94 nM | SANGER | |||
| SN12C | Growth Inhibition Assay | IC50=394.87 nM | SANGER | |||
| BHT-101 | Growth Inhibition Assay | IC50=395.92 nM | SANGER | |||
| DMS-114 | Growth Inhibition Assay | IC50=396.31 nM | SANGER | |||
| NCI-H1838 | Growth Inhibition Assay | IC50=405.05 nM | SANGER | |||
| HT | Growth Inhibition Assay | IC50=405.15 nM | SANGER | |||
| Caov-3 | Growth Inhibition Assay | IC50=406.19 nM | SANGER | |||
| LB2518-MEL | Growth Inhibition Assay | IC50=413.1 nM | SANGER | |||
| C3A | Growth Inhibition Assay | IC50=415.79 nM | SANGER | |||
| NCI-H226 | Growth Inhibition Assay | IC50=416.64 nM | SANGER | |||
| KMS-12-PE | Growth Inhibition Assay | IC50=418.06 nM | SANGER | |||
| RERF-LC-MS | Growth Inhibition Assay | IC50=425.05 nM | SANGER | |||
| OE33 | Growth Inhibition Assay | IC50=426.02 nM | SANGER | |||
| COLO-678 | Growth Inhibition Assay | IC50=427.18 nM | SANGER | |||
| NCI-H1963 | Growth Inhibition Assay | IC50=430.11 nM | SANGER | |||
| LAMA-84 | Growth Inhibition Assay | IC50=437.3 nM | SANGER | |||
| NCI-H2171 | Growth Inhibition Assay | IC50=440.67 nM | SANGER | |||
| U-266 | Growth Inhibition Assay | IC50=451.49 nM | SANGER | |||
| SNU-C2B | Growth Inhibition Assay | IC50=460.97 nM | SANGER | |||
| T47D | Growth Inhibition Assay | IC50=469.69 nM | SANGER | |||
| TE-6 | Growth Inhibition Assay | IC50=469.81 nM | SANGER | |||
| OVCAR-3 | Growth Inhibition Assay | IC50=470.41 nM | SANGER | |||
| C-4-II | Growth Inhibition Assay | IC50=472.56 nM | SANGER | |||
| EFM-19 | Growth Inhibition Assay | IC50=478.27 nM | SANGER | |||
| NCI-N87 | Growth Inhibition Assay | IC50=479.92 nM | SANGER | |||
| NCI-H187 | Growth Inhibition Assay | IC50=493.21 nM | SANGER | |||
| MDA-MB-157 | Growth Inhibition Assay | IC50=499.01 nM | SANGER | |||
| OPM-2 | Growth Inhibition Assay | IC50=501.81 nM | SANGER | |||
| COLO-320-HSR | Growth Inhibition Assay | IC50=513.16 nM | SANGER | |||
| RMG-I | Growth Inhibition Assay | IC50=513.98 nM | SANGER | |||
| IST-SL1 | Growth Inhibition Assay | IC50=517.42 nM | SANGER | |||
| UACC-893 | Growth Inhibition Assay | IC50=521.51 nM | SANGER | |||
| Becker | Growth Inhibition Assay | IC50=521.83 nM | SANGER | |||
| HPAF-II | Growth Inhibition Assay | IC50=527.24 nM | SANGER | |||
| HCC1954 | Growth Inhibition Assay | IC50=540.83 nM | SANGER | |||
| SNU-449 | Growth Inhibition Assay | IC50=548.01 nM | SANGER | |||
| NCI-H1563 | Growth Inhibition Assay | IC50=556.49 nM | SANGER | |||
| HDLM-2 | Growth Inhibition Assay | IC50=568.59 nM | SANGER | |||
| NCI-H69 | Growth Inhibition Assay | IC50=568.8 nM | SANGER | |||
| NCI-SNU-5 | Growth Inhibition Assay | IC50=570.15 nM | SANGER | |||
| NCI-H1792 | Growth Inhibition Assay | IC50=572.43 nM | SANGER | |||
| NCI-H2141 | Growth Inhibition Assay | IC50=573.69 nM | SANGER | |||
| KMOE-2 | Growth Inhibition Assay | IC50=576.32 nM | SANGER | |||
| NCI-H1155 | Growth Inhibition Assay | IC50=579.87 nM | SANGER | |||
| LK-2 | Growth Inhibition Assay | IC50=581.07 nM | SANGER | |||
| HCC1187 | Growth Inhibition Assay | IC50=591.47 nM | SANGER | |||
| EGI-1 | Growth Inhibition Assay | IC50=598.27 nM | SANGER | |||
| NCI-H1623 | Growth Inhibition Assay | IC50=604.6 nM | SANGER | |||
| MN-60 | Growth Inhibition Assay | IC50=605.37 nM | SANGER | |||
| JAR | Growth Inhibition Assay | IC50=625.95 nM | SANGER | |||
| HCC1419 | Growth Inhibition Assay | IC50=631.74 nM | SANGER | |||
| LU-165 | Growth Inhibition Assay | IC50=639.4 nM | SANGER | |||
| TYK-nu | Growth Inhibition Assay | IC50=662.55 nM | SANGER | |||
| DG-75 | Growth Inhibition Assay | IC50=665.83 nM | SANGER | |||
| PFSK-1 | Growth Inhibition Assay | IC50=678.08 nM | SANGER | |||
| MKN7 | Growth Inhibition Assay | IC50=689.95 nM | SANGER | |||
| DMS-153 | Growth Inhibition Assay | IC50=698.15 nM | SANGER | |||
| HTC-C3 | Growth Inhibition Assay | IC50=703.94 nM | SANGER | |||
| GCIY | Growth Inhibition Assay | IC50=725.12 nM | SANGER | |||
| NCI-H1092 | Growth Inhibition Assay | IC50=737.95 nM | SANGER | |||
| EB-3 | Growth Inhibition Assay | IC50=771.02 nM | SANGER | |||
| CAL-120 | Growth Inhibition Assay | IC50=777.52 nM | SANGER | |||
| BT-474 | Growth Inhibition Assay | IC50=778.81 nM | SANGER | |||
| L-428 | Growth Inhibition Assay | IC50=790.9 nM | SANGER | |||
| Mo-T | Growth Inhibition Assay | IC50=804.65 nM | SANGER | |||
| CAL-72 | Growth Inhibition Assay | IC50=840.67 nM | SANGER | |||
| AsPC-1 | Growth Inhibition Assay | IC50=855.26 nM | SANGER | |||
| U-118-MG | Growth Inhibition Assay | IC50=856.34 nM | SANGER | |||
| GCT | Growth Inhibition Assay | IC50=861.48 nM | SANGER | |||
| EKVX | Growth Inhibition Assay | IC50=871.87 nM | SANGER | |||
| NCI-H1755 | Growth Inhibition Assay | IC50=873.52 nM | SANGER | |||
| Capan-2 | Growth Inhibition Assay | IC50=898.11 nM | SANGER | |||
| NCI-H1793 | Growth Inhibition Assay | IC50=900.87 nM | SANGER | |||
| PLC-PRF-5 | Growth Inhibition Assay | IC50=901.35 nM | SANGER | |||
| M14 | Growth Inhibition Assay | IC50=905.27 nM | SANGER | |||
| SK-MEL-24 | Growth Inhibition Assay | IC50=914.35 nM | SANGER | |||
| SK-MM-2 | Growth Inhibition Assay | IC50=942.3 nM | SANGER | |||
| PC-3 | Growth Inhibition Assay | IC50=957.22 nM | SANGER | |||
| EW-24 | Growth Inhibition Assay | IC50=970.4 nM | SANGER | |||
| MDA-MB-175-VII | Growth Inhibition Assay | IC50=971.53 nM | SANGER | |||
| NCI-H1581 | Growth Inhibition Assay | IC50=981.31 nM | SANGER | |||
| THP-1 | Growth Inhibition Assay | IC50=989.42 nM | SANGER | |||
| NCI-H1993 | Growth Inhibition Assay | IC50=1.00076 μM | SANGER | |||
| DOK | Growth Inhibition Assay | IC50=1.01762 μM | SANGER | |||
| KINGS-1 | Growth Inhibition Assay | IC50=1.019 μM | SANGER | |||
| YT | Growth Inhibition Assay | IC50=1.04568 μM | SANGER | |||
| TGBC1TKB | Growth Inhibition Assay | IC50=1.06036 μM | SANGER | |||
| TCCSUP | Growth Inhibition Assay | IC50=1.23083 μM | SANGER | |||
| BEN | Growth Inhibition Assay | IC50=1.23742 μM | SANGER | |||
| KARPAS-45 | Growth Inhibition Assay | IC50=1.26763 μM | SANGER | |||
| COLO-829 | Growth Inhibition Assay | IC50=1.2748 μM | SANGER | |||
| UACC-62 | Growth Inhibition Assay | IC50=1.32145 μM | SANGER | |||
| NCI-H446 | Growth Inhibition Assay | IC50=1.33771 μM | SANGER | |||
| RO82-W-1 | Growth Inhibition Assay | IC50=1.34337 μM | SANGER | |||
| NCI-H2452 | Growth Inhibition Assay | IC50=1.37129 μM | SANGER | |||
| RL | Growth Inhibition Assay | IC50=1.42066 μM | SANGER | |||
| NCI-H1522 | Growth Inhibition Assay | IC50=1.43159 μM | SANGER | |||
| TGW | Growth Inhibition Assay | IC50=1.45942 μM | SANGER | |||
| HT55 | Growth Inhibition Assay | IC50=1.47168 μM | SANGER | |||
| KARPAS-299 | Growth Inhibition Assay | IC50=1.47841 μM | SANGER | |||
| KY821 | Growth Inhibition Assay | IC50=1.49429 μM | SANGER | |||
| MDA-MB-231 | Growth Inhibition Assay | IC50=1.53055 μM | SANGER | |||
| NCI-H82 | Growth Inhibition Assay | IC50=1.57026 μM | SANGER | |||
| MDA-MB-134-VI | Growth Inhibition Assay | IC50=1.61711 μM | SANGER | |||
| PANC-08-13 | Growth Inhibition Assay | IC50=1.63084 μM | SANGER | |||
| D-542MG | Growth Inhibition Assay | IC50=1.65305 μM | SANGER | |||
| LC-1F | Growth Inhibition Assay | IC50=1.66203 μM | SANGER | |||
| KP-N-YN | Growth Inhibition Assay | IC50=1.66343 μM | SANGER | |||
| MFE-280 | Growth Inhibition Assay | IC50=1.66812 μM | SANGER | |||
| S-117 | Growth Inhibition Assay | IC50=1.70504 μM | SANGER | |||
| NCI-H1573 | Growth Inhibition Assay | IC50=1.71733 μM | SANGER | |||
| BT-20 | Growth Inhibition Assay | IC50=1.74068 μM | SANGER | |||
| NCI-H28 | Growth Inhibition Assay | IC50=1.74078 μM | SANGER | |||
| CaR-1 | Growth Inhibition Assay | IC50=1.78324 μM | SANGER | |||
| LN-405 | Growth Inhibition Assay | IC50=1.80143 μM | SANGER | |||
| RT4 | Growth Inhibition Assay | IC50=1.84417 μM | SANGER | |||
| NCI-H889 | Growth Inhibition Assay | IC50=1.93886 μM | SANGER | |||
| SW1463 | Growth Inhibition Assay | IC50=1.95701 μM | SANGER | |||
| SCLC-21H | Growth Inhibition Assay | IC50=2.00624 μM | SANGER | |||
| CP66-MEL | Growth Inhibition Assay | IC50=2.01767 μM | SANGER | |||
| Hs-578-T | Growth Inhibition Assay | IC50=2.01782 μM | SANGER | |||
| HCC70 | Growth Inhibition Assay | IC50=2.05463 μM | SANGER | |||
| SW1088 | Growth Inhibition Assay | IC50=2.06931 μM | SANGER | |||
| NB6 | Growth Inhibition Assay | IC50=2.185 μM | SANGER | |||
| KU-19-19 | Growth Inhibition Assay | IC50=2.26917 μM | SANGER | |||
| A704 | Growth Inhibition Assay | IC50=2.29135 μM | SANGER | |||
| EVSA-T | Growth Inhibition Assay | IC50=2.30089 μM | SANGER | |||
| HCT-15 | Growth Inhibition Assay | IC50=2.35466 μM | SANGER | |||
| HuO-3N1 | Growth Inhibition Assay | IC50=2.36583 μM | SANGER | |||
| GMS-10 | Growth Inhibition Assay | IC50=2.39738 μM | SANGER | |||
| TGBC24TKB | Growth Inhibition Assay | IC50=2.41429 μM | SANGER | |||
| C2BBe1 | Growth Inhibition Assay | IC50=2.51328 μM | SANGER | |||
| SK-N-DZ | Growth Inhibition Assay | IC50=2.56592 μM | SANGER | |||
| SK-N-AS | Growth Inhibition Assay | IC50=2.73015 μM | SANGER | |||
| T98G | Growth Inhibition Assay | IC50=2.80401 μM | SANGER | |||
| DMS-79 | Growth Inhibition Assay | IC50=2.90988 μM | SANGER | |||
| SNU-475 | Growth Inhibition Assay | IC50=2.92572 μM | SANGER | |||
| COLO-792 | Growth Inhibition Assay | IC50=3.0287 μM | SANGER | |||
| YAPC | Growth Inhibition Assay | IC50=3.32832 μM | SANGER | |||
| UACC-812 | Growth Inhibition Assay | IC50=3.33738 μM | SANGER | |||
| Mewo | Growth Inhibition Assay | IC50=3.46086 μM | SANGER | |||
| COLO-824 | Growth Inhibition Assay | IC50=3.57378 μM | SANGER | |||
| SCC-9 | Growth Inhibition Assay | IC50=3.91309 μM | SANGER | |||
| CP50-MEL-B | Growth Inhibition Assay | IC50=3.98703 μM | SANGER | |||
| NCI-H345 | Growth Inhibition Assay | IC50=4.01174 μM | SANGER | |||
| COR-L88 | Growth Inhibition Assay | IC50=4.3958 μM | SANGER | |||
| RCM-1 | Growth Inhibition Assay | IC50=4.49272 μM | SANGER | |||
| JVM-3 | Growth Inhibition Assay | IC50=4.60618 μM | SANGER | |||
| ECC4 | Growth Inhibition Assay | IC50=4.76541 μM | SANGER | |||
| MDA-MB-453 | Growth Inhibition Assay | IC50=4.85134 μM | SANGER | |||
| SK-N-FI | Growth Inhibition Assay | IC50=5.22877 μM | SANGER | |||
| D-502MG | Growth Inhibition Assay | IC50=5.24262 μM | SANGER | |||
| NCI-H2227 | Growth Inhibition Assay | IC50=5.36231 μM | SANGER | |||
| DMS-53 | Growth Inhibition Assay | IC50=6.17106 μM | SANGER | |||
| LB771-HNC | Growth Inhibition Assay | IC50=6.46806 μM | SANGER | |||
| BB49-HNC | Growth Inhibition Assay | IC50=7.41791 μM | SANGER | |||
| MDA-MB-415 | Growth Inhibition Assay | IC50=7.57303 μM | SANGER | |||
| TALL-1 | Growth Inhibition Assay | IC50=7.9688 μM | SANGER | |||
| HCC1395 | Growth Inhibition Assay | IC50=8.15122 μM | SANGER | |||
| EFE-184 | Growth Inhibition Assay | IC50=8.37216 μM | SANGER | |||
| EC-GI-10 | Growth Inhibition Assay | IC50=8.68686 μM | SANGER | |||
| TT | Growth Inhibition Assay | IC50=9.06966 μM | SANGER | |||
| U-2-OS | Growth Inhibition Assay | IC50=9.81393 μM | SANGER | |||
| SHP-77 | Growth Inhibition Assay | IC50=9.9029 μM | SANGER | |||
| HCC1143 | Growth Inhibition Assay | IC50=10.4797 μM | SANGER | |||
| A498 | Growth Inhibition Assay | IC50=18.2976 μM | SANGER | |||
| LS-513 | Growth Inhibition Assay | IC50=18.6093 μM | SANGER | |||
| K052 | Growth Inhibition Assay | IC50=21.4418 μM | SANGER | |||
| SiHa | Growth Inhibition Assay | IC50=23.3371 μM | SANGER | |||
| RCC10RGB | Growth Inhibition Assay | IC50=30.0611 μM | SANGER | |||
| NCI-SNU-16 | Growth Inhibition Assay | IC50=31.1836 μM | SANGER | |||
| SNU-C1 | Growth Inhibition Assay | IC50=32.4175 μM | SANGER | |||
| C32 | Growth Inhibition Assay | IC50=32.872 μM | SANGER | |||
| SW684 | Growth Inhibition Assay | IC50=34.1561 μM | SANGER | |||
| SK-MEL-28 | Growth Inhibition Assay | IC50=35.5377 μM | SANGER | |||
| SK-MEL-1 | Growth Inhibition Assay | IC50=36.7547 μM | SANGER | |||
| NCI-H720 | Growth Inhibition Assay | IC50=41.9726 μM | SANGER | |||
| P31-FUJ | Growth Inhibition Assay | IC50=44.3284 μM | SANGER | |||
| NCI-H524 | Growth Inhibition Assay | IC50=48.8248 μM | SANGER | |||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 100 mg/mL
(172.41 mM)
Water : 100 mg/mL Ethanol : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 579.98 | Formula | C27H29NO11.HCl |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 25316-40-9 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | Adriamycin, NSC 123127, Hydroxydaunorubicin HCl | Smiles | CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O.Cl | ||
Mechanism of Action
| Targets/IC50/Ki |
AMPK
Topo II
(Tumor cells) |
|---|---|
| In vitro |
Doxorubicin, an antibiotic anthracycline, is commonly considered to exert its anti-tumor activity at two fundamental levels, altering DNA and producing free radicals to trigger apoptosis of cancer cells through DNA damage. Doxorubicin can block the synthesis of DNA by intercalating into the DNA strand, and inhibits DNA topoisomerase II (TOP2). Doxorubicin is most effective when cells are rapidly proliferating and expressing high levels of TOP2. Additionally, Doxorubicin can trigger apoptosis by producing ceramide (which prompts apoptosis by activating p53 or other downstream pathways such as JNK), the degradation of Akt by serine threonine proteases, the mitochondrial release of cytochrome c, increased FasL (death receptor Fas/CD95 ligand) mRNA production, and a greater production of free radicals. Pre-treatment with GSNO (nitrosoglutathione) suppresses the resistance in the doxorubicin-resistant breast cancer cell line MCF7/Dx, accompanied by enhanced protein glutathionylation and accumulation of doxorubicin in the nucleus. Doxorubicin induced G2/M checkpoint arrest are attributed to elevated cyclin G2 (CycG2) expression and phospho-modification of proteins in the ataxia telangiectasia mutated (ATM) and ATM and Rad3-related (ATR) signaling pathways. Doxorubicin inhibits AMP-activated protein kinase (AMPK), resulting in SIRT1 dysfunction, p53 accumulation, and increased cell death in mouse embryonic fibroblasts (MEFs) and cardiomyocytes, which can be further sensitized by pre-inhibition of AMPK. Doxorubicin elicits a marked heat shock response, and that either inhibition or silencing of heat shock proteins enhance the Doxorubicin apoptotic effect in neuroblastoma cells. Nanomolar Doxorubicin treatment of neuroblastoma cells causes dose-dependent over-ubiquitination of a specific set of proteins in the absence of measurable inhibition of proteasome, and loss of activity of ubiquitinated enzymes such as lactate dehydrogenase and α-enolase, the protein ubiquination patterns of which is similar to those with proteasome inhibitor, indicating that Doxorubicin may also exert its effect by damaging proteins. |
| In vivo |
In vivo, Doxorubicin in combination with adenoviral MnSOD (AdMnSOD) plus 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) has the greatest effect in decreasing the volumes of MB231 tumors and prolonging survival of mice. Although its use is limited by the chronic and acute toxic side effects it produces, Doxorubicin is essential in treating breast and oesophageal carcinomas, solid tumours in childhood, osteosarcomas, Kaposi's sarcoma, soft tissue sarcomas, and Hodgkin and non-Hodgkin lymphomas. |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | POU5F1 / SOX2 / NANOG ATG5 / ATG7 / SQSTM1 / LC3A-II / LC3B-II / p-mTOR P62 / LC3II p-AKT(T308) / p-Akt(S473) / Akt / p-PRAS40(T246) / PRAS40 / p-H2A.X(S139) |
|
27929731 |
| Growth inhibition assay | Cell viability |
|
25871400 |
| Immunofluorescence | LC3 |
|
24970805 |
| ELISA | OPN |
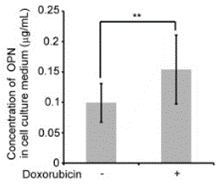
|
24963635 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06186700 | Active not recruiting | Breast Cancer Female |
Mansoura University |
December 25 2023 | Phase 2 |
| NCT05567601 | Not yet recruiting | Ovarian Cancer|AIDS-related Kaposi Sarcoma|Multiple Myeloma|Metastatic Breast Cancer |
Baxter Healthcare Corporation |
November 30 2023 | Phase 1 |
| NCT06088290 | Recruiting | Leiomyosarcoma |
PharmaMar |
September 21 2023 | Phase 2|Phase 3 |
| NCT05748834 | Recruiting | Breast Cancer |
SCRI Development Innovations LLC|Seagen Inc. |
July 24 2023 | Phase 2 |
| NCT05853120 | Recruiting | Sexually Transmitted Diseases |
Emory University|Centers for Disease Control and Prevention |
May 31 2023 | Phase 4 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
Why did the IC50 of it vary over time in our measurement?
Answer:
Sometimes the IC50 value might vary between different batch of compounds due to the slight differences in purity. If different IC50 was seen in the same batch of it, the problem may lies in the process of dissolvement. Make sure that you see no particles and the solution is clear with your stock solution.






































