research use only
Dacomitinib EGFR inhibitor
Cat.No.S2727
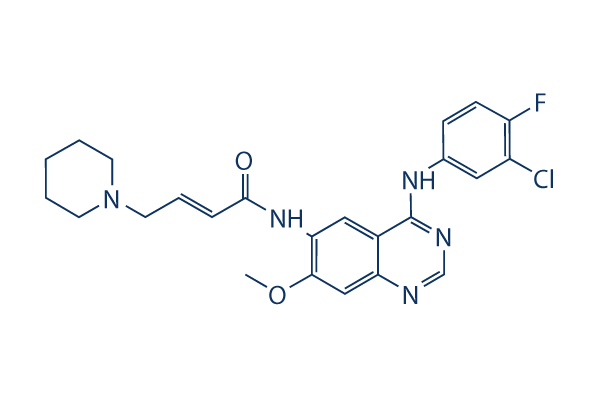
Chemical Structure
Molecular Weight: 469.94
Quality Control
| Related Targets | VEGFR PDGFR FGFR c-Met Src MEK CSF-1R FLT3 HER2 c-Kit |
|---|---|
| Other EGFR Inhibitors | Lazertinib (YH25448) Icotinib Hydrochloride Sunvozertinib AG-490 AG-1478 Canertinib (CI-1033) WZ4002 Poziotinib (NOV120101, HM781-36B) Rociletinib (CO-1686) Genistein |
Cell Culture, Treatment & Working Concentration
| Cell Lines | Assay Type | Concentration | Incubation Time | Formulation | Activity Description | PMID |
|---|---|---|---|---|---|---|
| PC9 cells | Function assay | 2 h | Inhibition of EGFR exon 19 deletion activating mutant phosphorylation in human PC9 cells after 2 hrs by fluorescence assay, IC50=0.63 nM | 23930994 | ||
| human LoVo cells | Function assay | 2 h | Inhibition of wild type EGFR phosphorylation in human LoVo cells after 2 hrs by fluorescence assay, IC50=0.011 μM | 23930994 | ||
| human NCI-H1975 cells | Function assay | 2 h | Inhibition of EGFR L858R/T970M double mutant phosphorylation in human NCI-H1975 cells after 2 hrs by fluorescence assay, IC50=0.042 μM | 23930994 | ||
| human NCI-H1975 cells | Proliferation assay | Antiproliferative activity against human NCI-H1975 cells assessed as growth inhibition, GI50=0.1233 μM | 26310890 | |||
| Sf9 | Function assay | 6 mins | Irreversible inhibition of GST-tagged ERBB1 (unknown origin) (Met-668 to Ala-1211 residues) expressed in baculovirus infected Sf9 insect cells assessed as reduction in Glu/Tyr copolymer phosphorylation after 6 mins by ELISA, IC50 = 0.006 μM. | 27491023 | ||
| NIH/3T3 | Function assay | 2 hrs | Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed by stimulation with EGF for 10 mins, IC50 = 0.006 μM. | 27491023 | ||
| Sf9 | Function assay | 6 mins | Irreversible inhibition of GST-tagged ERBB2 (unknown origin) (Ile-675 to Val-1256 residues) expressed in baculovirus infected Sf9 insect cells assessed as reduction in Glu/Tyr copolymer phosphorylation after 6 mins by ELISA, IC50 = 0.046 μM. | 27491023 | ||
| Sf9 | Function assay | 6 mins | Irreversible inhibition of GST-tagged ERBB4 (unknown origin) (Gly-259 to Gly-690 residues) expressed in baculovirus infected Sf9 insect cells assessed as reduction in Glu/Tyr copolymer phosphorylation after 6 mins by ELISA, IC50 = 0.074 μM. | 27491023 | ||
| Sf9 | Function assay | 30 mins | Irreversible inhibition of human recombinant GST-tagged JAK3 expressed in baculovirus infected Sf9 insect cells assessed as reduction in polyglutamic acid-tyrosine phosphorylation after 30 mins by ELISA, IC50 = 3.57 μM. | 27491023 | ||
| NIH/3T3 | Function assay | 30 mg/kg | 2 days | In vivo inhibition of full length human ERBB1 autophosphorylation transfected in NIH/3T3 cells implanted in mouse at 30 mg/kg, po qd for 2 days measured 24 hrs post last dose by Western blot analysis | 27491023 | |
| insect cells | Function assay | Inhibition of GST-tagged human EGFR catalytic domain expressed in insect cells, IC50 = 0.006 μM. | 28754471 | |||
| NCI-H1819 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1819 cells expressing wild type HER2 incubated for 72 hrs by MTS assay, IC50 = 0.029 μM. | 28754471 | ||
| insect cells | Function assay | Inhibition of GST-tagged human HER2 catalytic domain expressed in insect cells, IC50 = 0.0457 μM. | 28754471 | |||
| NCI-H1975 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human NCI-H1975 cells expressing EGFR T790M/L858R mutant incubated for 72 hrs by MTS assay, IC50 = 0.44 μM. | 28754471 | ||
| Click to View More Cell Line Experimental Data | ||||||
Solubility
|
In vitro |
DMSO
: 49 mg/mL
(104.26 mM)
Ethanol : 15 mg/mL Water : Insoluble |
Molarity Calculator
|
In vivo |
|||||
In vivo Formulation Calculator (Clear solution)
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such
as vortex, ultrasound or hot water bath can be used to aid dissolving.
Chemical Information, Storage & Stability
| Molecular Weight | 469.94 | Formula | C24H25ClFN5O2 |
Storage (From the date of receipt) | |
|---|---|---|---|---|---|
| CAS No. | 1110813-31-4 | Download SDF | Storage of Stock Solutions |
|
|
| Synonyms | PF299804,PF299 | Smiles | COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)NC(=O)C=CCN4CCCCC4 | ||
Mechanism of Action
| Targets/IC50/Ki |
EGFR
(Cell-free assay) 6.0 nM
ErbB2
(Cell-free assay) 45.7 nM
ErbB4
(Cell-free assay) 73.7 nM
|
|---|---|
| In vitro |
PF299804 is a specific inhibitor of the ERBB family of kinases. This compound inhibits EGFR signaling and induces apoptosis in the EGFR T790M-containing H3255 GR cell line. It is effective in -sensitive and NSCLC cell lines. This chemical inhibits the growth of H3255 and HCC827 cells engineered to express EGFR T790M. It inhibits EGFR phosphorylation in the presence of the T790M mutation. This agent is believed to irreversibly inhibit ERBB tyrosine kinase activity through binding at the ATP site and covalent modification of nucleophilic cysteine residues in the catalytic domains of ERBB family members. It shows significant growth-inhibitory effects in HER2-amplified gastric cancer cells (SNU216, N87), and it has lower 50% inhibitory concentration values compared with other EGFR tyrosine kinase inhibitors, including BIBW-2992, and CI-1033. This inhibitor induces apoptosis and G1 arrest and inhibits phosphorylation of receptors in the HER family and downstream signaling pathways including STAT3, AKT, and extracellular signal-regulated kinases (ERK) in HER2-amplified gastric cancer cells. It also blocks EGFR/HER2, HER2/HER3, and HER3/HER4 heterodimer formation as well as the association of HER3 with p85α in SNU216 cells. A recent research uses forty-seven human breast cancer and immortalized breast epithelial lines to evaluate the inhibition effects of this compound, the results indicate it preferentially inhibits growth of HER-2-amplified breast cancer cell lines than nonamplified lines (RR = 3.39, p < 0.0001). This chemical reduces the phosphorylation of HER2, EGFR, HER4, AKT, and ERK in the majority of sensitive lines. It exerts its anti-proliferative effect through a combined G0/G1 arrest and an induction of apoptosis. |
| Kinase Assay |
ELISA-Based ERBB Kinase Assay
|
|
The ERBB1, ERBB 2, and ERBB4 cytoplasmic fusion proteins are made by cloning the ERBB1 sequence (Met-668 to Ala-1211), ERBB2 (Ile-675 to Val-1256), and ERBB4 sequence (Gly-259 to Gly-690) into the baculoviral vector pFastBac using PCR. Proteins are expressed in baculovirusinfected Sf9 insect cells as GST fusion proteins. The proteins are purified by affinity chromatography using glutathione sepharose beads. Inhibition of ERBB tyrosine kinase activity is assessed using an ELISA-based receptor tyrosine kinase assay. Kinase reactions (50 mM HEPES, pH 7.4, 125 mM NaCl, 10 mM MgCl2, 100 μM sodium orthovanadate, 2 mM dithiothreitol, 20 μM ATP, this compound or vehicle control, and 1-5 nM GST-erbB per 50 μL of reaction mixture) are run in 96-well plates coated with 0.25 mg/mL poly-Glu-Tyr. The reactions are incubated for 6 minutes at room temperature while being shaken. Kinase reactions are stopped by removal of the reaction mixture, and then the wells are washed with wash buffer (0.1% Tween 20 in PBS). Phosphorylated tyrosine residues are detected by adding 0.2 μg/mL antiphosphotyrosine antibody (Oncogene Ab-4; 50 μL/well) coupled to horseradish peroxidase (HRP) diluted in PBS containing 3% BSA and 0.05% Tween 20 for 25 minutes while being shaken at room temperature. The antibody is removed, and plates are washed in wash buffer. HRP substrate (SureBlue3,3,5,5-tetramethyl benzidine or TMB) is added (50 μL per well) and incubated for 10-20 minutes while it is shaken at room temperature. The TMB reaction is stopped with the addition of 50 μL of stop solution (0.09 N H2SO4). The signal is quantified by measuring absorbance at 450 nm. IC50 values are determined for this compound using the median effect method.
|
|
| In vivo |
Orally administered PF299804 effectively inhibits growth of HCC827 Del/T790M xenografts. Low oral administration of this compound (15mg/kg) causes significant antitumor activity, including marked tumor regressions in a variety of human tumor xenograft models that express and/ or overexpress ERBB family members or contain the double mutation (L858R/T790M) in ERBB1 (EGFR). |
References |
|
Applications
| Methods | Biomarkers | Images | PMID |
|---|---|---|---|
| Western blot | pEGFR / EGFR / pERK / ERK / pAKT / AKT / p-mTOR / mTOR / pSTAT3 / STAT3 |
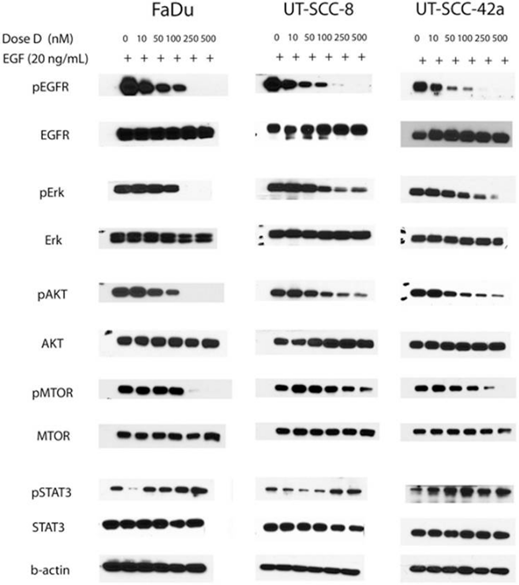
|
24853121 |
| Immunofluorescence | LC3 |
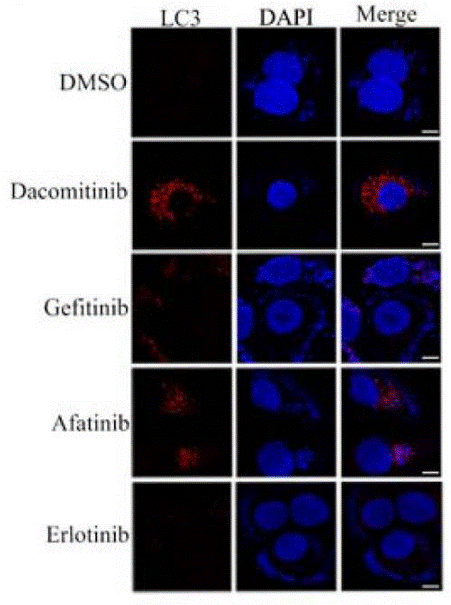
|
28366635 |
| Growth inhibition assay | Cell viability |
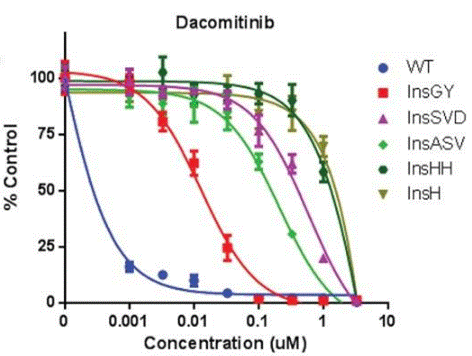
|
28363995 |
Clinical Trial Information
(data from https://clinicaltrials.gov, updated on 2024-05-22)
| NCT Number | Recruitment | Conditions | Sponsor/Collaborators | Start Date | Phases |
|---|---|---|---|---|---|
| NCT06075615 | Not yet recruiting | Carcinoma Non-Small-Cell Lung |
Pfizer |
September 1 2024 | -- |
| NCT06321510 | Not yet recruiting | Lung Cancer |
Pfizer |
April 1 2024 | -- |
| NCT04609319 | Active not recruiting | Lung Cancer |
Pfizer |
January 23 2021 | -- |
| NCT03865446 | Completed | Severe Hepatic Impairment |
Pfizer |
April 5 2019 | Phase 1 |
| NCT02382796 | Completed | NSCLC |
Pfizer |
July 10 2015 | Phase 2 |
| NCT02268747 | Unknown status | Skin Squamous Cell Cancer |
Fondazione IRCCS Istituto Nazionale dei Tumori Milano |
November 2014 | Phase 2 |
Tech Support
Tel: +1-832-582-8158 Ext:3
If you have any other enquiries, please leave a message.
Frequently Asked Questions
Question 1:
I would like to know whether the in vivo formulation you recommend for it is suitable for oral administration?
Answer:
S2727 in 1% DMSO+30% polyethylene glycol+1% Tween 80 at 10mg/ml is a homogeneous suspension, and it was fine for oral gavage. When preparing the solution, please dissolve it in DMSO clearly first. If it dissolves not readily, please sonicate and warm it at about 45-50℃ for a while to help dissolving. Then add PEG 300 and Tween 80. After they mixed well, dilute with water. Then it will become a homogeneous suspension.






































